Constrictive pericarditis (CP) is a rare complication after lung transplantation (LTx), described in small case series after double lung transplantation.1,2 Its cause is unknown and many theories are proposed. In our cohort, we observed four cases of this rare condition one of them after single lung procedure, which is the first case described in the literature. Our aim is to describe these cases as well discuss about its etiology and diagnosis.
The first case was a 41-year-old male who underwent bilateral LTx for bronchiectasis due to IgA deficiency in October 2008. He was diagnosed with atypical mycobacterial infection one year later. After one year, he presented rapid onset of dyspnea and signs of congestive heart failure. Echocardiogram showed pericardial effusion with signs of cardiac tamponade. Pericardial drainage was performed but he developed refractory cardiogenic shock and subsequently died 48h after surgery. Autopsy showed an important pericardial thickening and attributed CP as the cause of death.
The second case was a 59 years-old male patient with sequential bilateral lung transplantation due to idiopathic pulmonary fibrosis in February 2013. His postoperative was uneventful but 5 months after he was diagnosed with rectal adenocarcinoma, stage I, treated exclusively with radiotherapy. He was also submitted some months after to a Nissen's fundoplication due to gastric esophageal reflux. He also presented dengue fever. Two years after the LTx, the patient presented with symptoms of right heart failure and the echocardiogram showed pericardial thickening and small pericardial effusion. The cardiac magnetic resonance imaging (MRI) showed signs of constrictive pericarditis. He was submitted to pericardiectomy and epicardiectomy with waffle procedure technique. He recovered his normal function and he is in follow up without symptoms.
The third case was a 44-year-old male who underwent bilateral LTx for bronchiectasis due to tuberculosis sequelae in November 2014. The postoperative was uneventful but nine months after he presented with deterioration in respiratory function. Echocardiogram showed a large pericardial effusion and cardiac MRI showed no signs of pericardial thickening. Pericardial drainage was performed with 800ml of hemorrhagic fluid and prompt resolution of symptoms. Six months later he developed the same symptoms and without pericardial effusion on echocardiogram. MRI showed a thickened pericardium up to 4mm thick. Pericardiectomy was performed, the patient recovered his previous status and remains asymptomatic.
The last case was a 59 years-old male with idiopathic pulmonary fibrosis received a single left lung transplantation in December 2014. The procedure was performed by left postero-lateral thoracotomy without CPB and the anastomosis technique was conventional with pericardial window around pulmonary veins and ischemic time of 240min. The postoperative period was uneventful, with one episode of asymptomatic rejection and Nissen's fundoplication after one year due to gastric esophageal reflux. Two years after the transplant the patient showed acute but progressive dyspnea and signs of right heart failure. No signs of rejection or infection were detected. There were minimal pericardial effusion and pericardial thickening on chest computed tomography scan (CT-scan) and the echocardiogram showed left ventricular ejection fraction (LVEF) of 63%, atypical movement of ventricular septa, minimal pericardial effusion with pericardial thickening without signs of restriction. Cardiac MRI identified restriction on right ventricular filling and a circumferential thickened pericardium of 5mm. Cardiac catheterization showed equalization of pressures in all cardiac chambers confirming the hypothesis of CP. The patient underwent a median sternotomy and a phrenic-to-phrenic pericardiectomy with epicardiectomy without cardiopulmonary bypass. He was discharged after 17 days and in his follow up there is no complication 15 months after surgery. The specimen confirmed the diagnosis of CP, with pericardial fibrous thickening with areas of fibrin deposition on the surface and some blood extravasation. The post-operative was uneventful with improvement of dyspnea and the patient recovered his regular activities for two years. The echocardiograms performed in this period showed normal LVEF and no signs of constriction. Then, he was diagnosed with pulmonary embolism and major depression with severe impairment of pulmonary function. He was sent to palliative care treatment and died one month after.
Constrictive pericarditis is a fibrous thickening of the pericardium compressing the heart and interfering in its filling. It is related to cardiac surgery, radiotherapy, rheumatological disturbances and tuberculosis. However, half of all cases are idiopathic or after viral infection. Its incidence after cardiac procedures ranges between 0.2 and 2.4%.3 The incidence in our cohort after lung transplantation is 1.1% which is little higher than the only incidence reported in the literature of 0.4%.4
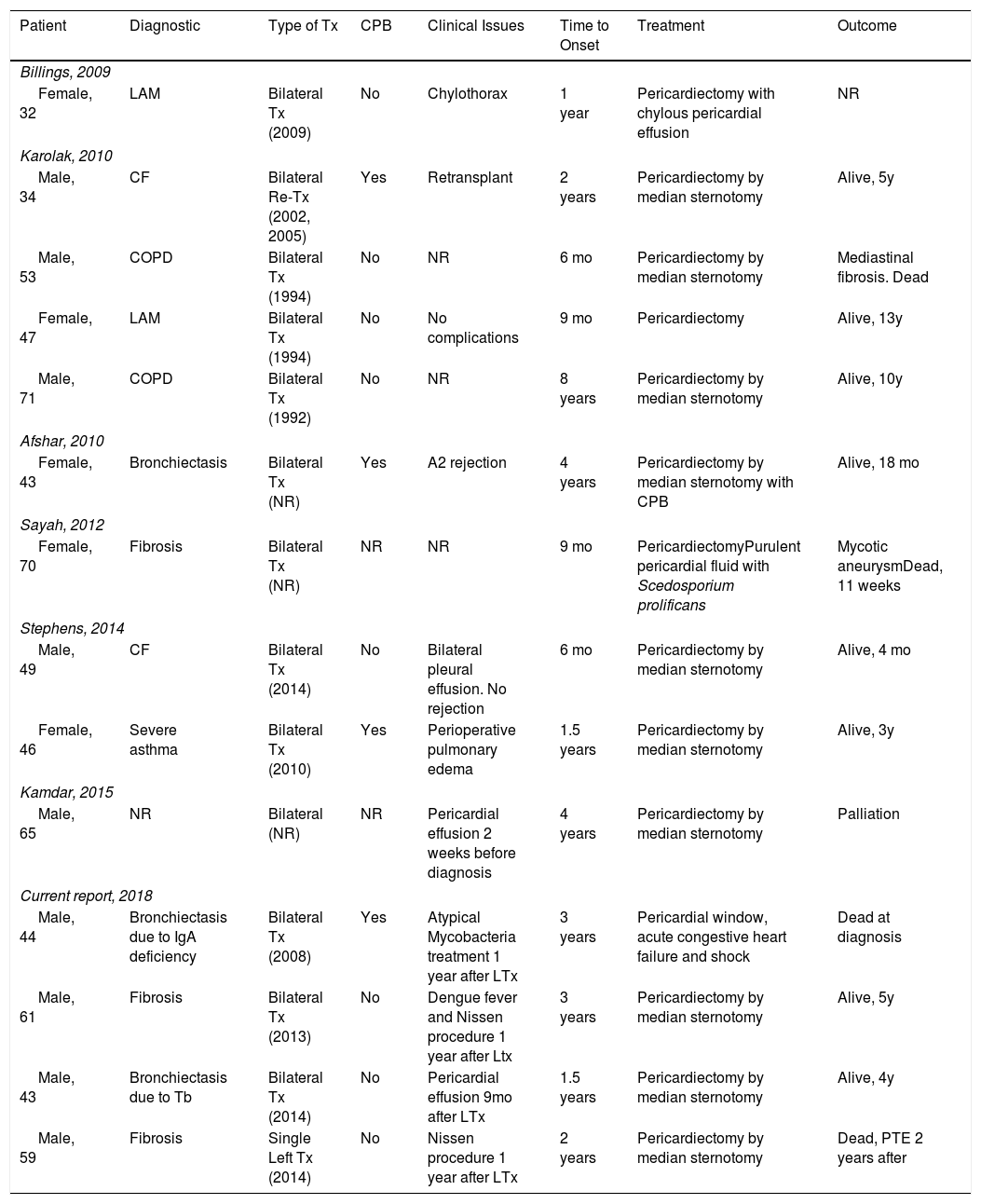
We performed an extensive literature search about this topic. Billings et al. were the first to describe this complication after LTx.5 After them, few reports and case series were published. There are only 15 cases reported worldwide. Table 1 resumes the main characteristics of these cases. All cases were bilateral and our one was the first unilateral (single left LTx). Interestingly only about 25% required cardiopulmonary bypass showing that cardiac manipulation was not related to the development of this condition. This is a relatively chronic disease with time to onset from the LTx to the development of symptoms varying between 6 months and 8 years.
Characteristics of CP Patients in Reports. CPB (Cardiopulmonary Bypass), Tb (Tuberculosis), Tx (Transplantation), Re-Tx (Retransplantation), PTE (Pulmonary Thromboembolism), CF (Cystic Fibrosis), LAM (Lymphangioleiomyomatosis), COPD (Chronic Obstructive Pulmonary Disease), NR (Not Reported), y (Year), mo (Month).
| Patient | Diagnostic | Type of Tx | CPB | Clinical Issues | Time to Onset | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Billings, 2009 | |||||||
| Female, 32 | LAM | Bilateral Tx (2009) | No | Chylothorax | 1 year | Pericardiectomy with chylous pericardial effusion | NR |
| Karolak, 2010 | |||||||
| Male, 34 | CF | Bilateral Re-Tx (2002, 2005) | Yes | Retransplant | 2 years | Pericardiectomy by median sternotomy | Alive, 5y |
| Male, 53 | COPD | Bilateral Tx (1994) | No | NR | 6 mo | Pericardiectomy by median sternotomy | Mediastinal fibrosis. Dead |
| Female, 47 | LAM | Bilateral Tx (1994) | No | No complications | 9 mo | Pericardiectomy | Alive, 13y |
| Male, 71 | COPD | Bilateral Tx (1992) | No | NR | 8 years | Pericardiectomy by median sternotomy | Alive, 10y |
| Afshar, 2010 | |||||||
| Female, 43 | Bronchiectasis | Bilateral Tx (NR) | Yes | A2 rejection | 4 years | Pericardiectomy by median sternotomy with CPB | Alive, 18 mo |
| Sayah, 2012 | |||||||
| Female, 70 | Fibrosis | Bilateral Tx (NR) | NR | NR | 9 mo | PericardiectomyPurulent pericardial fluid with Scedosporium prolificans | Mycotic aneurysmDead, 11 weeks |
| Stephens, 2014 | |||||||
| Male, 49 | CF | Bilateral Tx (2014) | No | Bilateral pleural effusion. No rejection | 6 mo | Pericardiectomy by median sternotomy | Alive, 4 mo |
| Female, 46 | Severe asthma | Bilateral Tx (2010) | Yes | Perioperative pulmonary edema | 1.5 years | Pericardiectomy by median sternotomy | Alive, 3y |
| Kamdar, 2015 | |||||||
| Male, 65 | NR | Bilateral (NR) | NR | Pericardial effusion 2 weeks before diagnosis | 4 years | Pericardiectomy by median sternotomy | Palliation |
| Current report, 2018 | |||||||
| Male, 44 | Bronchiectasis due to IgA deficiency | Bilateral Tx (2008) | Yes | Atypical Mycobacteria treatment 1 year after LTx | 3 years | Pericardial window, acute congestive heart failure and shock | Dead at diagnosis |
| Male, 61 | Fibrosis | Bilateral Tx (2013) | No | Dengue fever and Nissen procedure 1 year after Ltx | 3 years | Pericardiectomy by median sternotomy | Alive, 5y |
| Male, 43 | Bronchiectasis due to Tb | Bilateral Tx (2014) | No | Pericardial effusion 9mo after LTx | 1.5 years | Pericardiectomy by median sternotomy | Alive, 4y |
| Male, 59 | Fibrosis | Single Left Tx (2014) | No | Nissen procedure 1 year after LTx | 2 years | Pericardiectomy by median sternotomy | Dead, PTE 2 years after |
In the literature, only one case was reported after fungal infection.6 We excluded infection causes for pericarditis in our cases by culture and pathology analysis. One of our cases had bronchiectasis due to tuberculosis as cause for transplantation and other case had atypical mycobacteria treated after LTx. None of them showed infection as cause for pericarditis with no evidences of granuloma or presence of bacilli in the specimen. The first case, performed due to Lymphangioleiomyomatosishad chylothorax as complication after LTx and pericardial chylous effusion were found during pericardiectomy.7 It was not possible to relate a cause factor in the others cases reported suggesting an idiopathic manifestation of disease.
Manipulation of the pericardium has been considered as a potential risk factor for CP, including use of powdered gloves as a possibility.4 Bilateral procedures even without median opening of the pericardial sac, requires mediastinal shifting to hilum exposure and minimal manipulation of the pericardium around pulmonary vessels and bronchus. If a Clamshell incision is used it is possible to observe pericardial stretching sometimes leading to heart compression interfering in its filling with hypotension. In cases like this, we have adopted a technique to open the pericardial sac to alleviate the heart, pulling it up outside the normal position promoting better exposure of the hilum especially to the left side. In none of our bilateral cases we had to open the pericardium. In a single lung transplant the pericardium manipulation is even smaller and is only around the hilum vessels.8 As almost all cases reported in the literature we could not be able to identify a risk factor to its development in our cohort. The pathological specimen only showed typical features of pericarditis without granulomas or signs of infection.
The severity of the outcome requires rapid suspicious in every patient after lung transplantation. The definitive diagnosis relies on MRI showing thickening of pericardium and epicardium and cardiac catheterization with equalization of end diastolic pressure in all cardiac chambers, characteristic dip and plateau of ventricular diastolic pressure and resultant ventricular interdependence.9 Pericardiectomy and cardiac decortication must be ready performed in cases of progressive disease. Our first case had delayed diagnosis with severe shock after pericardial drainage. This patient died two days after and autopsy showed CP. The subsequent three patients, including this one, with similar symptoms had prompt suspicious with effective diagnosis and were submitted to success surgical treatment. The technique of pericardiectomy was by median sternotomy without cardiopulmonary bypass. Only one case in the literature required CPB to perform pericardiectomy.2 The thickened pericardium was removed phrenic-to-phrenic laterally and from the diaphragm to the aorta. Epicardiectomy was performed by subtotal decortication of the anterior and lateral faces of the heart. This is the crucial step of the surgery when sometimes the separation of the epicardium from the myorcardium is almost impossible. In cases like this, a Waffle procedure can be performed to avoid the risk of accidents.10 In these series, CP was related to worst outcome with death or palliation in three patients with a mortality rate of almost 20%. The effective treatment with an aggressive surgery as the pericardiectomy on the other hand was responsible for a good response with long survival rate.
This is the first case of CP after a single lung transplantation. Since there was no cardiopulmonary assistance and minimal pericardial manipulation, idiopathic or multifactorial causes should be involved. The most important is the prompt diagnosis to assure ideal surgical treatment to avoid fatal outcome.