Pneumonia by SARS-CoV-2 may induce a wide spectrum of pulmonary sequelae, with diffuse interstitial pulmonary involvement that may lead to pulmonary fibrosis being of note.
Viruses may be an environmental trigger of autoimmunity in genetically predisposed individuals, with viral infections being known modulators of immune response. In this sense, viral infection by SARS-CoV-2 could produce a Th1 inflammatory response which would present common zones with the pathogenesis of hypersensitivity pneumonitis (HP) leading to an entity of similar characteristics after the initial infection, although no case series have been described to date.1
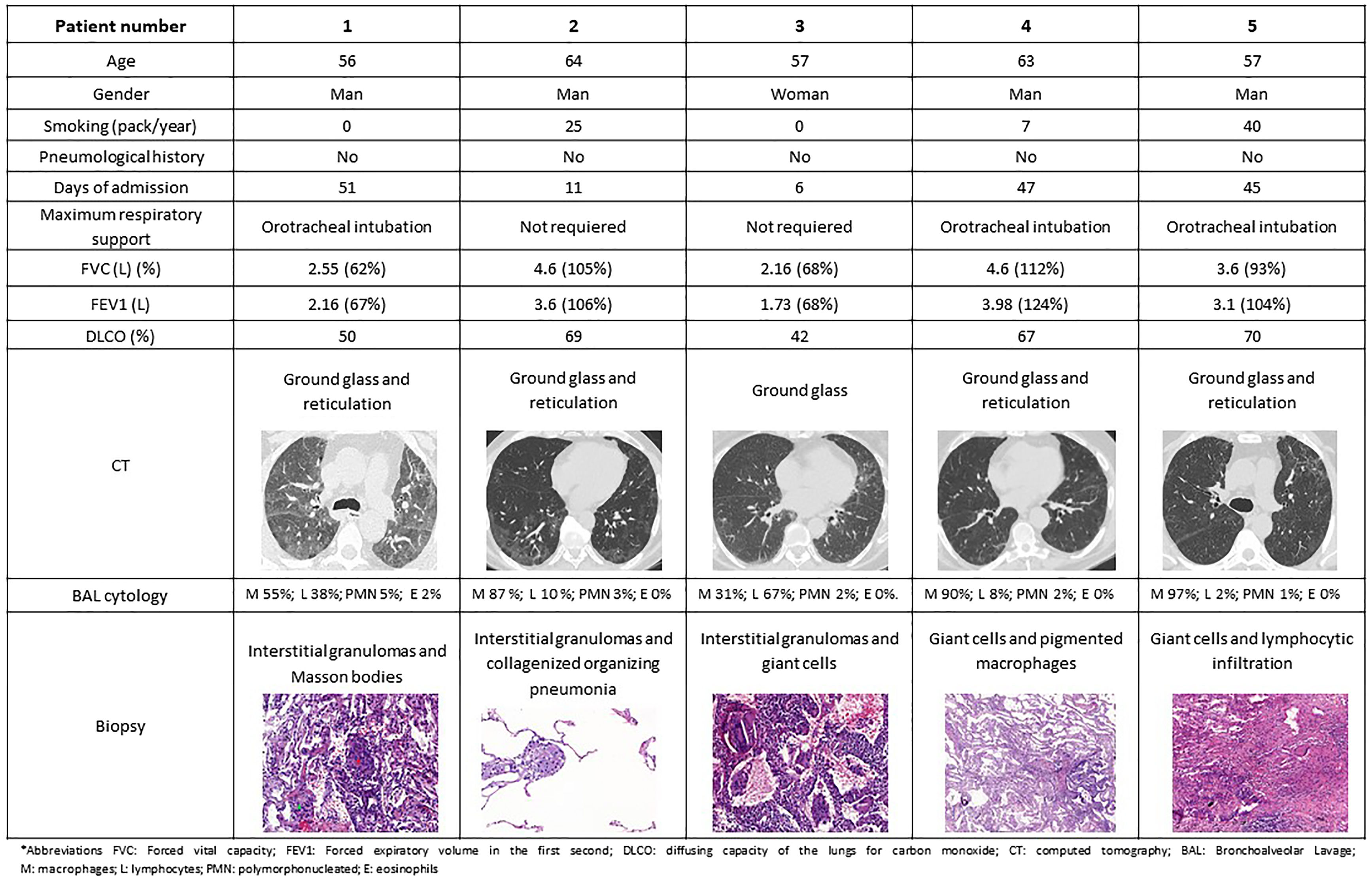
Between May 2020 and December 2021, 2586 patients were admitted in our hospital due to COVID-19 pneumonia and all were followed in the respiratory outpatient clinics. During the follow-up, all the patients underwent a high-resolution computerized tomography (HRCT) and lung function tests within three months of hospital discharge. Sixty-eight cases presented sequelae of interstitial lung involvement, requiring the performance of a transbronchial cryobiopsy in order to investigate the aetiology. A number of 5 out 68 cryobiopsies showed findings mimicking HP (7.35%) and these cases were carefully reviewed by the expert multidisciplinary interstitial lung disease team and other causes of HP were excluded.2 Regarding these 5 patients, the mean age was 59 years (p25–p75 57–63 years), 4 were men (4/5), 3 were ex-smokers and arterial hypertension was the most frequent comorbidity (2/5). None had presented previous lung disease. Regarding their admission for COVID pneumonia, all were diagnosed by polymerase chain reaction, 3 required orotracheal intubation and admission to the intensive care unit (ICU), while the other 2 only required low-flow oxygen therapy in a conventional ward. All the included patients were on wide spectrum systemic antibiotics during their admission encompassing ceftriaxone, azithromycin or meropenem. Four of them also received hydroxychloroquine (for 5 days) and lopinavir/ritonavir (for 2 days), and another patient received 2 doses of tocilizumab according to the local recommendations during that period. While pneumonitis has been described in context of hydroxychloroquine and ritonavir, it is very unlikely that in the presented clinical frame these drugs were the cause of the described histological findings. In the HRCT performed, the predominant findings were patchy pulmonary opacities and bilateral ground glass images with peripheral linear and reticular densities. Pulmonary function test in all these patients showed a non-obstructive ventilatory disorder with a reduction in the diffusing capacity of the lungs for carbon monoxide (DLCO). A common biopsy finding was patchy pneumonitis with interstitial granulomas. Lymphocyte infiltrate was present in 3 cases, while giant cell clusters presented also in 3 cases (Fig. 1).
Although the hypothesis of the relationship between SARS-CoV-2 and HP had previously been suggested by other authors,3 to our knowledge this is the first study to describe a cases serie showing histological involvement compatible with HP in patients with diffuse interstitial disease as a sequela of COVID pneumonia. The agents that trigger the immune reaction and the disease varies and more than 100 causes have been described, including a viral aetiology. In fact, other ribonucleic acid viruses such as influenza or syncytial respiratory virus have been described as being involved in the aetiology of HP.
HP is a lung disease caused by immune response to a variety of inhaled antigens, producing inflammation at the bronchioalveolar level in genetically predisposed individuals. Progression of the sensitization to HP requires the accumulation of CD4+ Th1 cells in the lung, creating a proinflammatory microenvironment that precipitates the disease.4 During the cell response derived from SARS-COV-2 infection, once exposed to the antigen, the Th0 cells mainly polarize to Th1, leading to the release of IFN-c to eliminate the virus. In predisposed individuals, the cellular response of the T cells may be suboptimal, deteriorated or excessive in patients with COVID-19, perpetuating a proinflammatory pulmonary status.5
Thus, taking into account that SARS-CoV-2 launches a mechanism of T1 cell response and HP is produced by predominantly T1 cellular response, it is likely that after the infection, common inflammatory pathways are activated and produce HP-like histological findings as observed in the present study. Thus, this subgroup of patients may benefit from a more specific treatment, in terms of dosage and duration of oral corticostereoids and immunosuppressants that may be closer to the management of HP.
Conflict of InterestIO declares to have received honoraria in the last three years for participating as a speaker in meetings sponsored by Astrazeneca, Boehringuer-Ingelheim, Chiesi, and Novartis and as a consultant for Astrazeneca, GlaxoSmithKlein, Puretech and Sanofi. He received financial aid from Astrazeneca, Bial and Chiesi for congress attendance and has received grants from Sanofi for research projects.