Exposure to feather bedding may be an unnoticed cause of hypersensitivity pneumonitis (HP) and idiopathic pulmonary fibrosis (IPF). Thus, an in-depth clinical study of the diagnosis of patients with suspected HP and IPF is required in order to determine their etiologies. The objective of the present study is to raise awareness of HP and pulmonary fibrosis due to exposure to feather bedding, and to study the prevalence and describe long-term outcomes.
MethodsWe describe a series of 33 patients diagnosed with HP and pulmonary fibrosis due to feather bedding exposure and followed over a 10-year period. The patients were from a subgroup of 127 individuals with HP undergoing in-depth evaluation using a diagnostic protocol at a regional referral center.
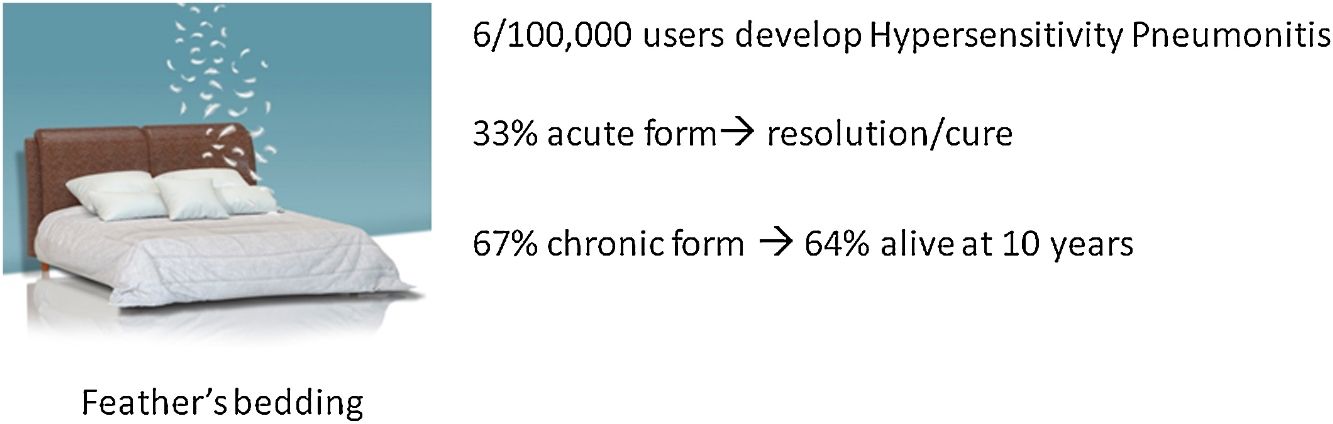
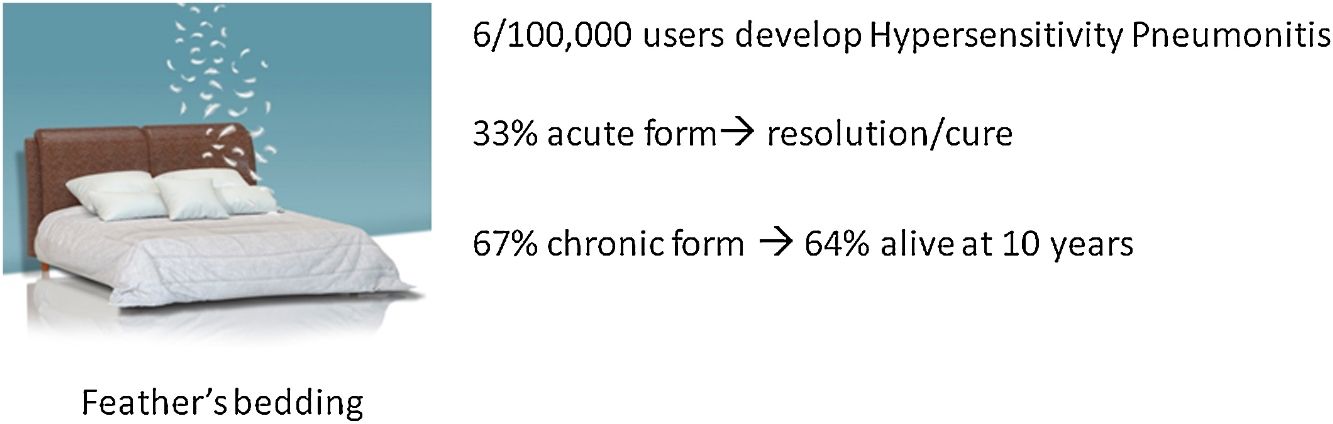
ResultsEleven (33%) patients were clinically diagnosed with acute HP and 22 (67%) with chronic HP. Ten (45%) chronic HP patients showed a high resolution computed tomography (HRCT) pattern of usual interstitial pneumonia (UIP) with suspected IPF. The prevalence of HP was 6.2/100 000 feather bedding users (compared with 54.6 per 100 000 bird-breeders). The survival rates of patients over the 10-year period was 100% for acute HP and 64% for chronic HP.
ConclusionsIn a series of HP patients, the diagnosis was attributed to feather bedding exposure in 26%. UIP pattern on HRCT was present in nearly half of the chronic cases. The survival of patients with chronic HP at ten years was 64%, despite avoiding further exposure.
La exposición a la ropa de cama rellena de plumas puede ser una causa que pase inadvertida de neumonitis por hipersensibilidad (NH) y fibrosis pulmonar idiopática (FPI). Por lo tanto, se requiere un estudio clínico en profundidad durante el proceso diagnóstico de pacientes con sospecha de NH e FPI para determinar sus etiologías. El objetivo del presente estudio es crear conciencia sobre la NH y la fibrosis pulmonar debidas a la exposición a la ropa de cama rellena de plumas, y estudiar la prevalencia y describir los resultados a largo plazo.
MétodosDescribimos una serie de 33 pacientes diagnosticados con NH y fibrosis pulmonar debido a la exposición a la ropa de cama rellena de plumas y en seguimiento durante un período de 10 años. Los pacientes pertenecían a un subgrupo de 127 individuos con NH a los cuales se les estaba realizando una evaluación en profundidad utilizando un protocolo de diagnóstico en un centro de referencia regional.
ResultadosA 11 pacientes (33%) se les diagnosticó de NH aguda y a 22 (67%) de NH crónica. Diez de los pacientes con NH crónica (45%) mostraron un patrón de neumonía intersticial usual (NIU) en la tomografía computarizada de alta resolución (TCAR), con sospecha de FPI. La prevalencia de NH fue de 6,2/100.000 usuarios de ropa de cama rellena de plumas (en comparación con el 54,6 por cada 100.000 criadores de aves). Las tasas de supervivencia de los pacientes durante el período de 10 años fueron del 100% para la NH aguda y del 64% para la NH crónica.
ConclusionesEn una serie de pacientes con NH, el diagnóstico se atribuyó a la exposición a la ropa de cama rellena de plumas en un 26%. El patrón NIU en el TCAR estaba presente en casi la mitad de los casos crónicos. La supervivencia de los pacientes con NH crónica a los 10 años fue del 64%, a pesar de evitar posteriores exposiciones.
The diagnosis of hypersensitivity pneumonitis (HP) is challenging depending on the degree of clinical suspicion, based on the exposure to antigens known to induce this inflammatory reaction. The appearance of a characteristic pattern observed in high resolution computed tomography (HRCT) images can raise the probability of diagnosis, and a predominance of lymphocytes in bronchoalveolar lavage (BAL) can also suggest the presence of HP.1,2 While the presence of specific immunoglobulin G antibodies in the serum (ssIgG) of patients indicates exposure to this antigen,3 a positive specific inhalation challenge (SIC) with the suspected antigen is also of diagnostic value especially to establish the etiology and confirm the diagnosis.2,4–8 In fact, the sensitivity and specificity of the SIC for the diagnosis of HP by exposure to avian and/or fungal antigens have been reported by our group to be 85% and 86% respectively,9 figures very similar to those obtained by other autors.4,8 Finally, evidence of improvement and avoidance of further exposure favors the diagnosis of HP.10
The temporal relationship between overt and/or subtle/occult presence of environmental factors and symptoms may raise the suspicion of the development of HP.11,12 Several recent studies have reported that a lack of awareness of infrequent environmental factors may explain, in part, the apparent lack of an attributable antigen in 25%–60% of patients with HP.10,13,14 Although exposure to birds is a well-known causative factor for HP, to date there are no prospective studies on HP due to contact with occult avian or fungal antigens in feathers within the home, for example in feather duvets and pillows. There have been sporadic reports of HP induced by feather bedding,2,15–17 but there are no established diagnostic criteria and the prevalence of this entity remains unknown. In addition, a recent study found that at least 43% of patients diagnosed with idiopathic pulmonary fibrosis (IPF) actually had chronic HP, and that half of the cases were due to occult exposure to feather bedding.6
The main objective of this study was to raise awareness of the diagnosis of HP due to exposure to concealed feathers at home. Diagnostic criteria are proposed. To our knowledge, this is the first report of an epidemiologic study combined with a description of the long-term outcome of a series of well-defined patients with HP caused by feather bedding.
Material and MethodsStudy DesignThe study population comprised patients diagnosed with HP caused exclusively by contact with feather bedding, selected from an ongoing prospective study undertaken to evaluate the exposure of environmental factors as potential causative factor/s for new onset interstitial lung disease (ILD) referred to our center. Patients were followed up long-term over the study period (January 2004 to December 2013). All the patients included in this series were diagnosed with HP using the criteria by Vasakova et al.9 displayed in Table 1. The mean follow-up was calculated from the beginning of symptoms to the last visit. The study was approved by our institution's Ethics Committee.
Criteria for Diagnosis of HP (Using the Criteria of Vasakova et al.4).
| Exposure to an Antigen Known to Be a Cause of HP, Plus Clinical Characteristics and HRCT Manifestations, and | All (n=33) | Acute (n=11) | Chronic (n=22) |
|---|---|---|---|
| (a) SLB/cryobiopsy with acute definite HP # | 6/33 (18%)a | 2/11 (18%) | 4/22 (18%) |
| (b) SLB/cryobiopsy with acute compatible or with CHP AND ssIgG positive and/or BAL >20 | 13/33 (39%) | 3/11 (27%) | 10/22 (45%) |
| (c) ssIgG positive and SIC positive | 14/33b (42%) | 6/11c (54%) | 8/22d (36%) |
HP, hypersensitivity pneumonitis; SLB, surgical lung biopsy; ssIgG+, serum specific IgG; L, lymphocytes; CHP, chronic hypersensitivity pneumonitis; BAL, bronchoalveolar lavage; SIC, positive specific inhalation challenge.
The criteria used to diagnose HP following the ones described by Vasakova et al.,9 are displayed in Table 1 (left column).
Criteria for HP Diagnosis Due to Feather Bedding (Table 2)The criteria were: (1) no significant previous and/or present exposure to birds or to other known environmental factors which may cause HP, (2) history of exposure to concealed feathers, (3) positive SIC and/or ssIgG, and, in the case of fungus, isolation of the same fungus in the feathers obtained from the patient's duvet or pillow.
Diagnostic Criteria for Causality of HP Due to Exposure to Feather Bedding.
| Patients Previously Diagnosed as HP, Without Other Significant Exposure to Antigenic Producers of HP and | All (n=33) | Acute (n=11) | Chronic (n=22) |
|---|---|---|---|
| SIC positivea and/or ssIgG positive plus positive isolation of the same fungus in the feathers obtained from the patient's duvet or pillow | 33/127 (26%) | 11/33 (33%) | 22/33 (66%) |
A standardized questionnaire on ILD was used, and medical records were collected (Appendix). Briefly, the initial clinical manifestations were recorded as well as the subsequent symptoms; the clinical history of agents known to produce HP was recorded, and ssIgG to a panel of bird feathers, bird serum (goose, pigeon, parrot, parakeet, canary) goose feathers and fungus (Aspergillus, Penicillium, Mucor) was determined using an ELISA method2 (see Appendix). Table 3 shows all the clinical interventions and the tests performed and also the results. Pulmonary function tests were carried out according to the current recommendations.18,19 HRCT of the chest was performed with inspiratory and expiratory sequences, and the patterns and distribution of abnormalities were discussed in multidisciplinary dynamic discussions (MDD) and reviewed by three clinicians from Vall d’Hebron Hospital (FM, AV, IO) and GR. BAL was performed in accordance with the ERS recommendations.20 SIC was performed (see methodology below) only in consenting patients with a forced vital capacity (FVC)>50% and diffusion lung capacity for carbon monoxide (DLCO)>40%. A pool of mixed feathers was used as antigens, and feathers collected from the patient's bedding were obtained by the patients themselves and each one cultured for fungi.
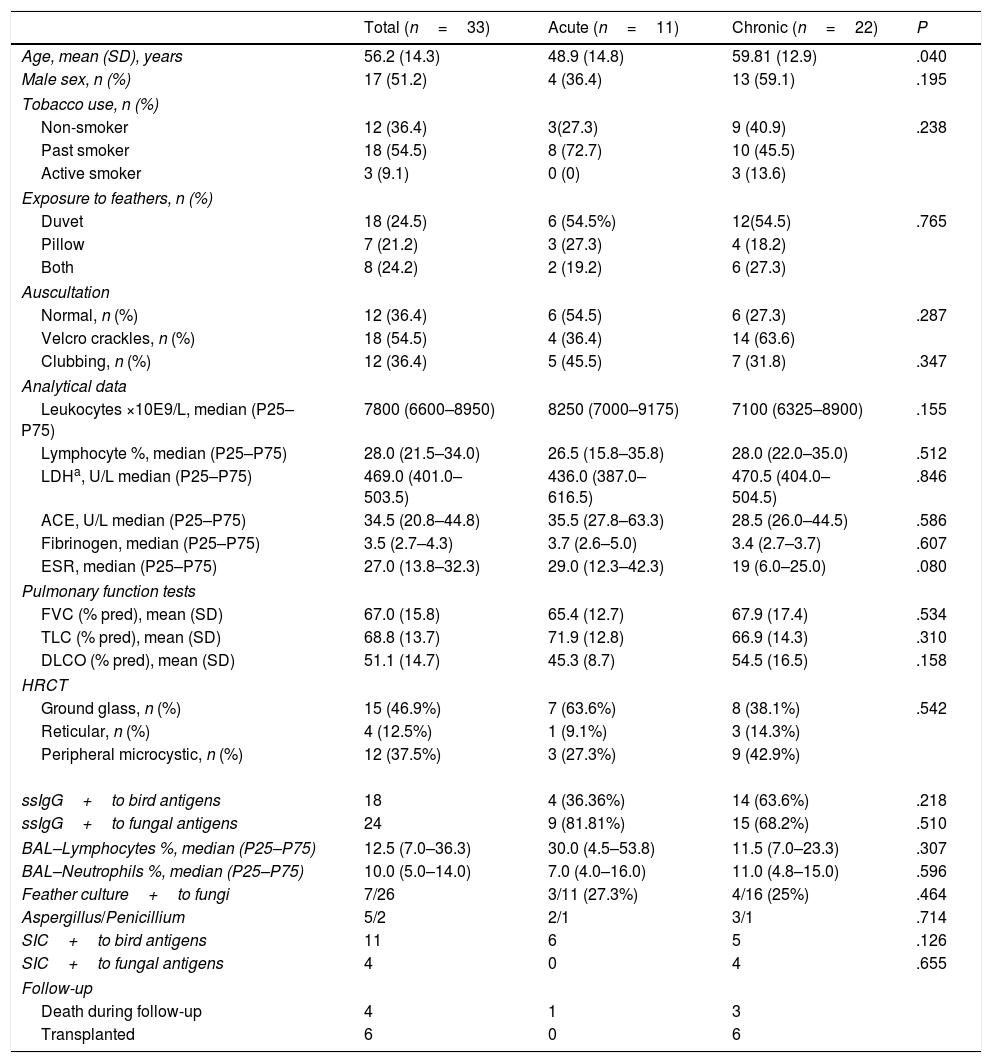
Clinical and Demographic Characteristics of 33 Patients With Acute or Chronic Hypersensitivity Pneumonitis Due to Exposure to Feather Bedding.
| Total (n=33) | Acute (n=11) | Chronic (n=22) | P | |
|---|---|---|---|---|
| Age, mean (SD), years | 56.2 (14.3) | 48.9 (14.8) | 59.81 (12.9) | .040 |
| Male sex, n (%) | 17 (51.2) | 4 (36.4) | 13 (59.1) | .195 |
| Tobacco use, n (%) | ||||
| Non-smoker | 12 (36.4) | 3(27.3) | 9 (40.9) | .238 |
| Past smoker | 18 (54.5) | 8 (72.7) | 10 (45.5) | |
| Active smoker | 3 (9.1) | 0 (0) | 3 (13.6) | |
| Exposure to feathers, n (%) | ||||
| Duvet | 18 (24.5) | 6 (54.5%) | 12(54.5) | .765 |
| Pillow | 7 (21.2) | 3 (27.3) | 4 (18.2) | |
| Both | 8 (24.2) | 2 (19.2) | 6 (27.3) | |
| Auscultation | ||||
| Normal, n (%) | 12 (36.4) | 6 (54.5) | 6 (27.3) | .287 |
| Velcro crackles, n (%) | 18 (54.5) | 4 (36.4) | 14 (63.6) | |
| Clubbing, n (%) | 12 (36.4) | 5 (45.5) | 7 (31.8) | .347 |
| Analytical data | ||||
| Leukocytes ×10E9/L, median (P25–P75) | 7800 (6600–8950) | 8250 (7000–9175) | 7100 (6325–8900) | .155 |
| Lymphocyte %, median (P25–P75) | 28.0 (21.5–34.0) | 26.5 (15.8–35.8) | 28.0 (22.0–35.0) | .512 |
| LDHa, U/L median (P25–P75) | 469.0 (401.0–503.5) | 436.0 (387.0–616.5) | 470.5 (404.0–504.5) | .846 |
| ACE, U/L median (P25–P75) | 34.5 (20.8–44.8) | 35.5 (27.8–63.3) | 28.5 (26.0–44.5) | .586 |
| Fibrinogen, median (P25–P75) | 3.5 (2.7–4.3) | 3.7 (2.6–5.0) | 3.4 (2.7–3.7) | .607 |
| ESR, median (P25–P75) | 27.0 (13.8–32.3) | 29.0 (12.3–42.3) | 19 (6.0–25.0) | .080 |
| Pulmonary function tests | ||||
| FVC (% pred), mean (SD) | 67.0 (15.8) | 65.4 (12.7) | 67.9 (17.4) | .534 |
| TLC (% pred), mean (SD) | 68.8 (13.7) | 71.9 (12.8) | 66.9 (14.3) | .310 |
| DLCO (% pred), mean (SD) | 51.1 (14.7) | 45.3 (8.7) | 54.5 (16.5) | .158 |
| HRCT | ||||
| Ground glass, n (%) | 15 (46.9%) | 7 (63.6%) | 8 (38.1%) | .542 |
| Reticular, n (%) | 4 (12.5%) | 1 (9.1%) | 3 (14.3%) | |
| Peripheral microcystic, n (%) | 12 (37.5%) | 3 (27.3%) | 9 (42.9%) | |
| ssIgG+to bird antigens | 18 | 4 (36.36%) | 14 (63.6%) | .218 |
| ssIgG+to fungal antigens | 24 | 9 (81.81%) | 15 (68.2%) | .510 |
| BAL–Lymphocytes %, median (P25–P75) | 12.5 (7.0–36.3) | 30.0 (4.5–53.8) | 11.5 (7.0–23.3) | .307 |
| BAL–Neutrophils %, median (P25–P75) | 10.0 (5.0–14.0) | 7.0 (4.0–16.0) | 11.0 (4.8–15.0) | .596 |
| Feather culture+to fungi | 7/26 | 3/11 (27.3%) | 4/16 (25%) | .464 |
| Aspergillus/Penicillium | 5/2 | 2/1 | 3/1 | .714 |
| SIC+to bird antigens | 11 | 6 | 5 | .126 |
| SIC+to fungal antigens | 4 | 0 | 4 | .655 |
| Follow-up | ||||
| Death during follow-up | 4 | 1 | 3 | |
| Transplanted | 6 | 0 | 6 | |
Normal values, LDH: 208–378UI/L, Fibrinogen: 2.3–6.1g/L, ECA: 13–63U/L.
% pred, percent of predicted value; ACE: angiotensin-converting enzyme; CHP, chronic hypersensitivity pneumonitis; DLCO, diffusion lung capacity for carbon monoxid corrected by Hb; HRCT, high-resolution computed tomography; HP, hypersensitivity pneumonitis; LDH, lactate dehydrogenase; SLB, surgical lung biopsy; TLC, total lung capacity; UIP, usual interstitial pneumonia; SIC, specific inhalation challenge.
The histopathological criteria were the ones described by Vasakova et al.9
Specific Inhalation Challenge5–7 (Appendix)Briefly, patients were requested to inhale 2mL of the suspected antigen (feathers or fungus, depending on ssIgG positivity) diluted to 1/100 (0.01mg/mL). The FVC in 1s (FEV1), DLCO, and temperature were recorded at baseline, 20min after inhalation, and every hour thereafter for the next 8h. Leukocyte count, chest X-ray, and O2 saturation measurements by pulse oximetry were performed before inhalation and 8h after. When the test proved negative it was repeated at 1/10 concentration 1 or 2 more days. Positivity criteria are shown in Appendix.
Treatment and Follow upAll patients were instructed to avoid further exposure and received oral prednisone at a dose ranging from 10 to 20mg/24h until pulmonary function tests normalized or were maintained if normalization was not achieved.
Patients were followed every 4 months with pulmonary function test. Survival was considered from the onset of HP symptoms to the last follow-up. The date of death was obtained from the patient's clinical history or with a phone call to their relatives. All lung transplantations were performed at our center.
Use of Feather Bedding in the General Population in BarcelonaDuring the mandatory medical check-up, we prospectively asked 400 consecutive healthcare professionals from our hospital whether they were currently using feather bedding. We put the same question to 1000 consecutive patients with bronchopulmonary diseases other than HP and IPF seen in the primary care setting.
Statistical AnalysisCategorical variables are expressed as absolute numbers and their corresponding percentages while continuous variables with a normal distribution are shown as means and standard deviations, and as medians and 25th to 75th percentiles in the case of those with a non-normal distribution. Demographic and clinical variables of patients with acute versus chronic HP were compared using the chi-square test for categorical variables (or fisher's exact test when one of the expected effects was below 5) and the Mann–Whitney U test for continuous variables.
For the survival analysis, we used the date of onset of respiratory symptoms and the date of death or lung transplant. Kaplan–Meier curves were performed to estimate the overall survival and the values after stratifying by disease stage at diagnosis and HRCT criteria for UIP. Differences in 10-year survival rates between the groups and their 95% confidence intervals (95% CI) were calculated. To overcome the difference in age between disease stage groups, we charted the survivor functions for the two categories adjusting for age by scaling to age 60 (the mean age in the chronic form). The Spanish web-assisted estimation of relative survival (WAERES) interface was used to calculate expected survival functions for the general population.21 The analysis was conducted using Stata 12.1 (StataCorp, College Station, TX, USA).
ResultsAmong the 127 patients with HP prospectively diagnosed at our center between 2004 and 2013, the condition was attributed to the use of feather bedding in 33 (26%). The condition was acute in 11 (33%) patients and chronic in 22 (67%). The clinical, demographic and diagnostic test results are shown in Table 3. There were no significant differences in any of the variables between acute and chronic patients, with the exception of age (acute patients being younger, P<.04). In 10 out of 22 (45%) chronic HP patients with a HRCT pattern of UIP, IPF was initially suspected.6
Test Results: Tables 1–3The antigen source was a feather duvet in 18 patients, a feather pillow in seven, and a combination of the two in eight. All the 33 patients underwent ssIgG testing to bird's sera, feathers and fungus extracts: 18/33 (54%) were positive to avian antigens, 24/33 (72%) to fungal antigens, and 13/33 (39%) to both. BAL was performed in 30/33 (90%) patients, revealing a lymphocyte count of at least 20% in 13 (43%) patients (6 acute and 7 chronic HP). Lung tissue was obtained from 20 patients: 15 by surgical lung biopsy (SLB), three by cryobiopsy, two through lung explants (both patients had previous SLB). The pathological review showed 6 inflammatory and 14 fibrotic patterns (Tables 1–3).
Feather cultures were performed in 26/33 (79%) cases, being positive in 7/26 (27%) (Aspergillus spp. in 5 and Penicillium spp. in 2).
SIC was proposed to all 33 patients. Sixteen patients underwent the challenge, and 17 patients did not (14 due to worsening lung function at the time of SIC, two who were unable to spend five days away from home, and one who refused to do the test). Eleven were positive to bird serum (six acute and five chronic HP) and four were positive to fungus (all in chronic form).
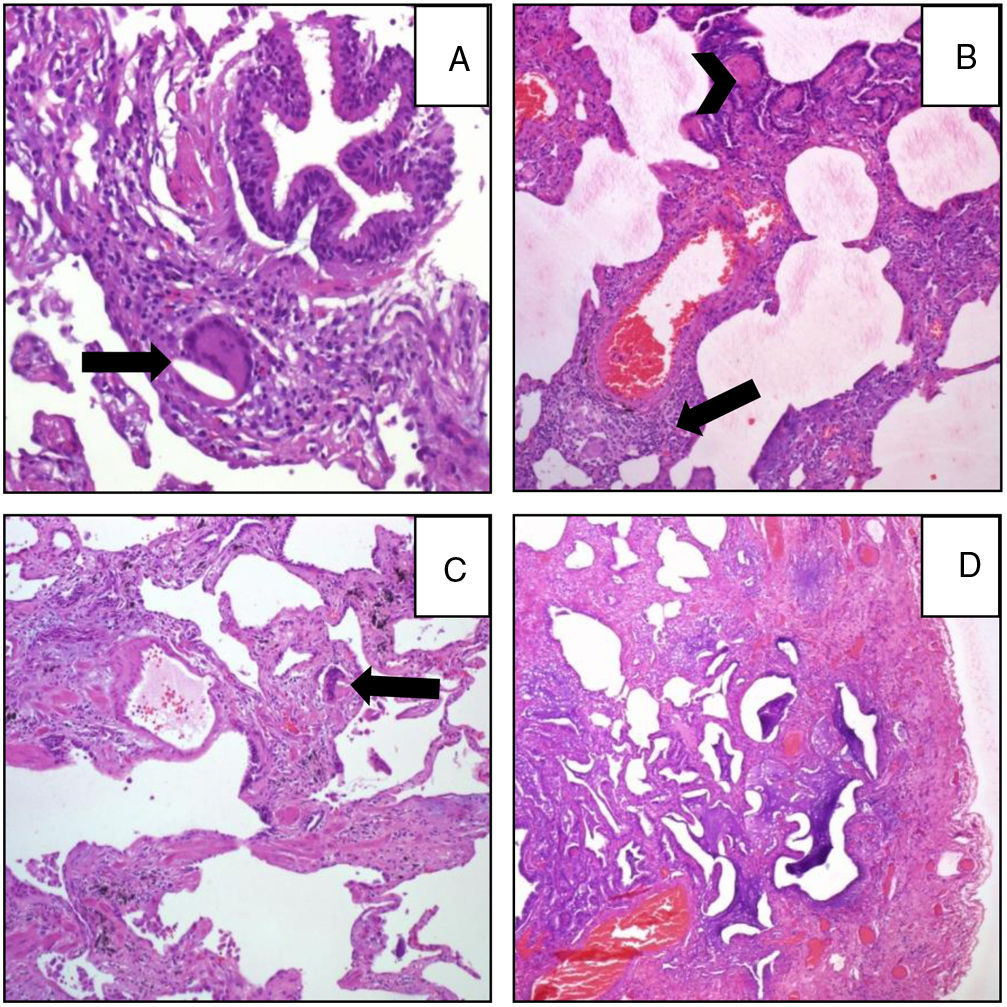
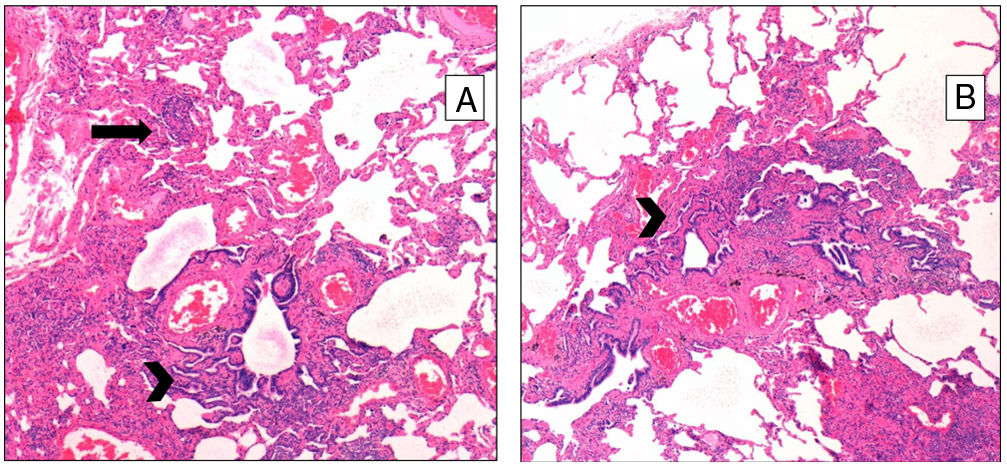
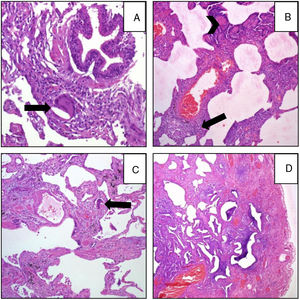
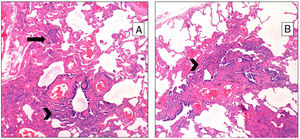
Pathological FindingsFigs. 1 and 2 show examples of pathological findings. This study used a temporal classification of acute and chronic HP.9
A (case 19): Acute: peribronchiolar chronic inflammatory infiltrate with an interstitial giant cell (arrow); B (case 9): acute: interstitial chronic inflammatory infiltrate with a poorly formed granuloma (arrow) and peribronchiolar metaplasia (arrow-head); C (case 9): chronic HP: the same case, four years later: explant specimen showing airway-centered interstitial fibrosis with an interstitial giant cell (arrow); D (case 17): chronic HP: advanced fibrosis with microscopic honeycombing.
Thirty per cent of the healthcare professionals questioned, and also 30% of the primary care patients with bronchopulmonary diseases other than HP and IPF, reported current use of feather bedding. Nine percent of healthcare professionals reported contact with birds in their homes for more than 5h per week.2
Epidemiological StudyAssessing epidemiological criteria, eight out of 33 of our patients with HP due to feather bedding lived inside our hospital's reference area (our hospital is the only one in this area of the city). Given that 30% of the 427,248 inhabitants of our reference area (www.bcn.es/estadistica) use feather bedding, that is, 128,174 inhabitants, the prevalence of HP due to concealed feathers can be estimated to be 6.2/100 000 feather bedding users (or 1.9/100 000 inhabitants) over a 10-year period. Over this same period, 21 patients were diagnosed with bird breeder's disease (BBD) in our area. Taking into account that 9% of the population of our area reported more than 5h contact with birds per week2 (that is, a total of 38,452 bird breeders), the prevalence of BBD is 54.6/100 000 bird breeders, or 4.9/100 000 inhabitants in our area.
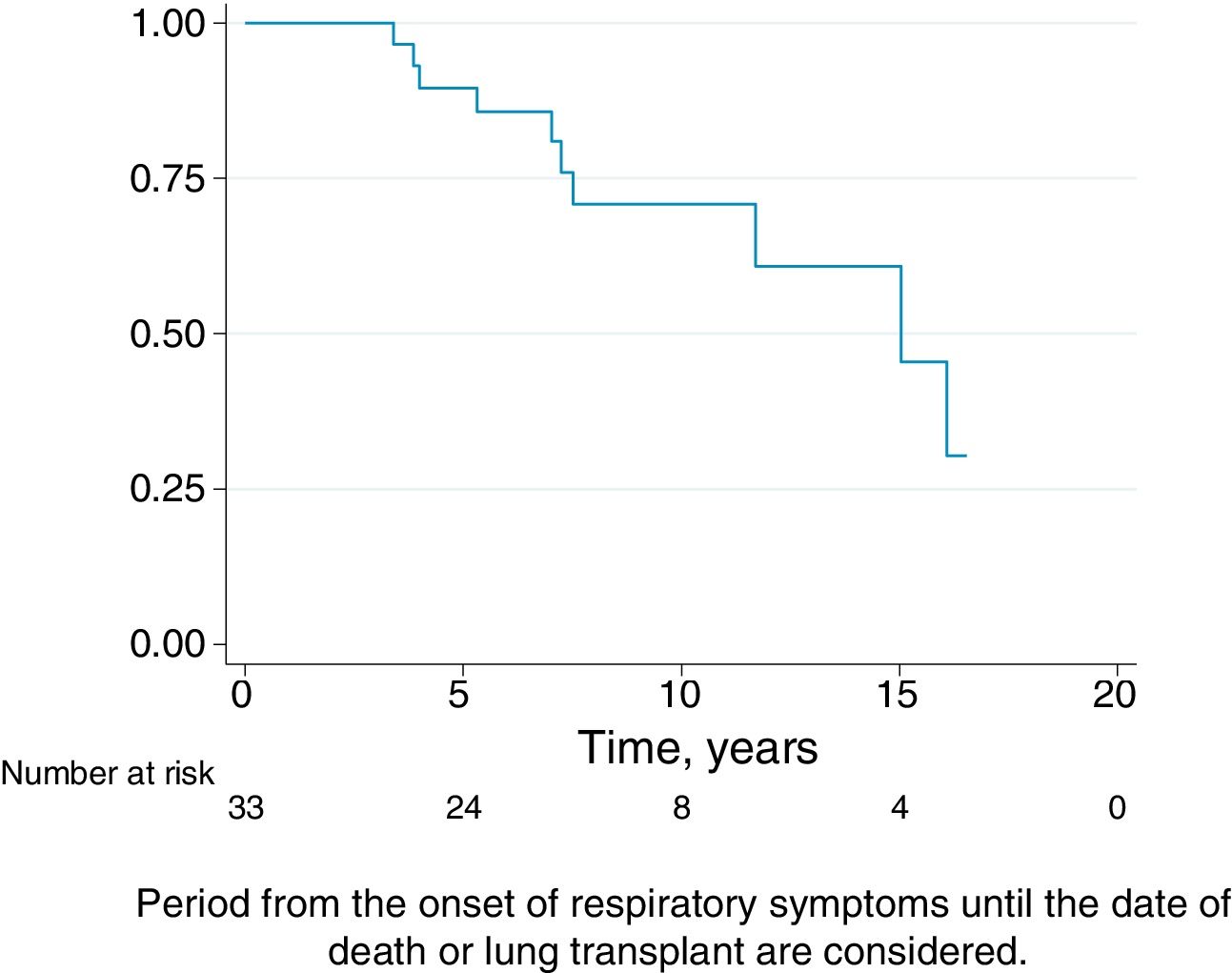
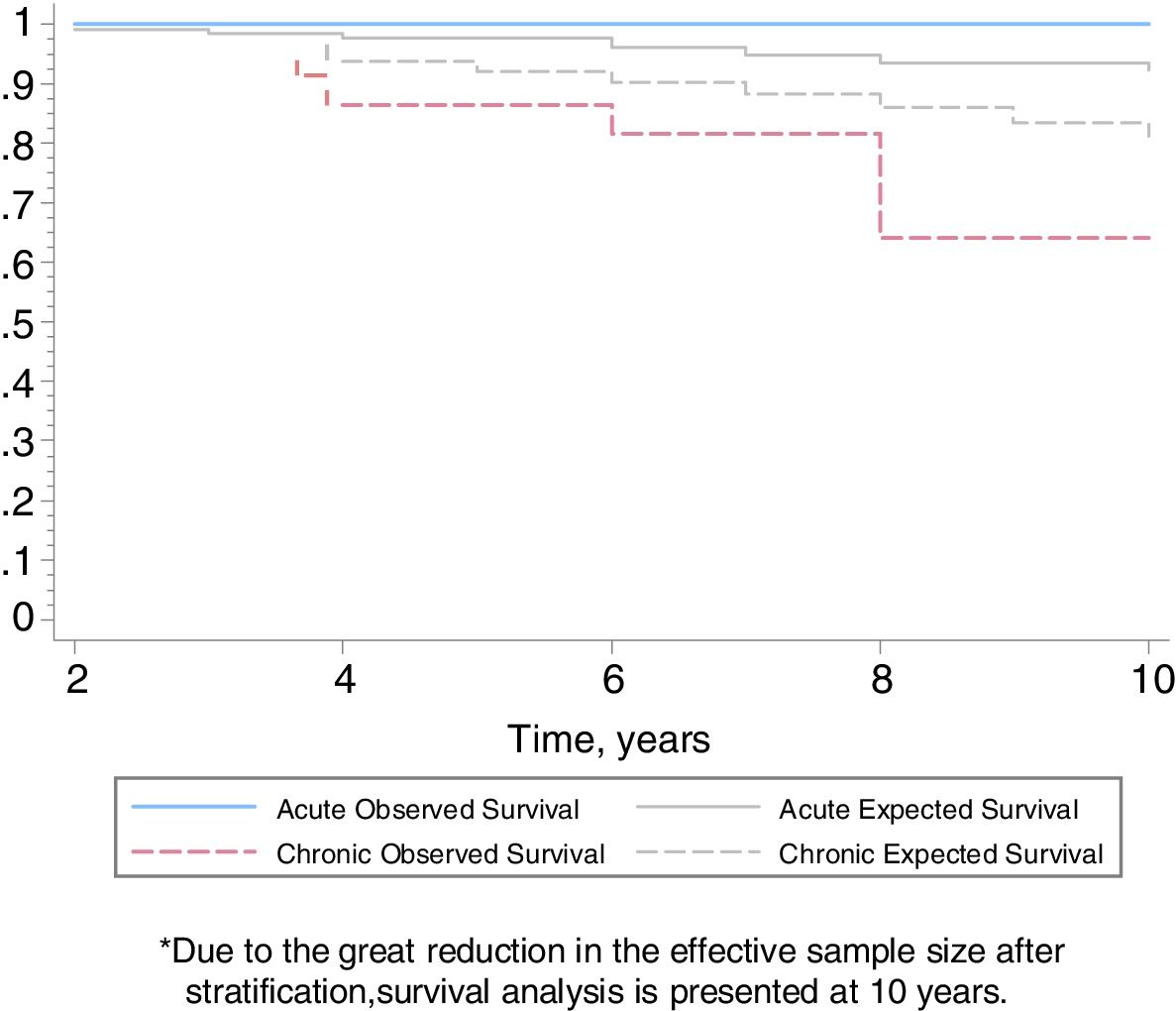
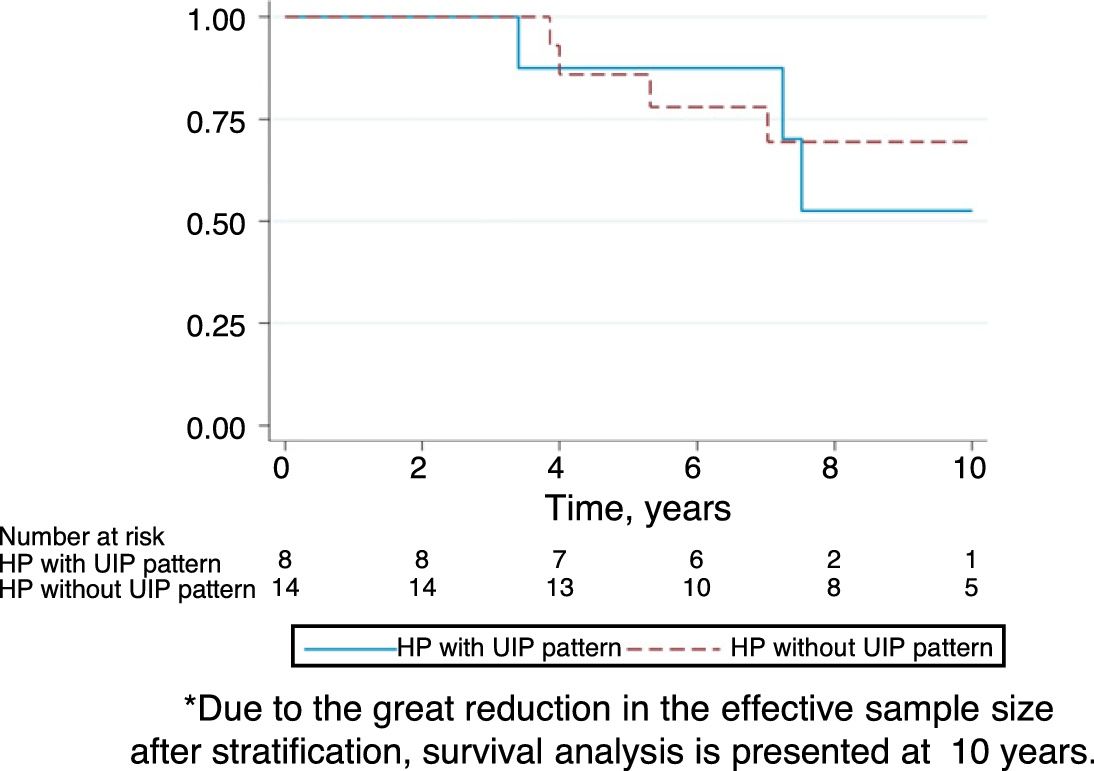
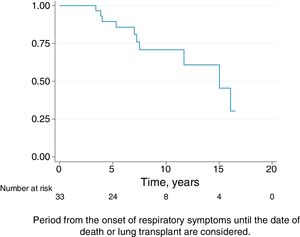
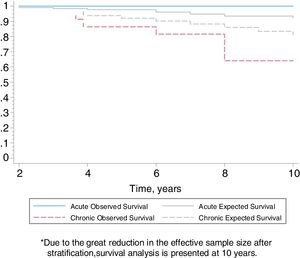
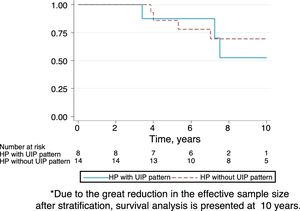
Follow-upThe median period of follow-up since symptom onset was 7.1 (4.5–9.5) years, and median follow-up period from the first visit was 4.4 (2.0–6.0) years. During follow-up, four patients died and six underwent lung transplantation (Table 3) and Fig. 3. The cause of death was respiratory failure in two patients (one with tuberculosis), one myocardial infarction, and one of unknown cause. The mean (SD) survival from symptom onset was 12.6 (5.8) years, with cumulative survival rates of 89.5% (95% CI: 77.8–100.0) at five years and 71% (95% CI: 52.3–89.5) at 10 years (Fig. 3). Survival analysis stratified according to the disease stage at diagnosis showed a difference in 10-year survival rate of 36% (95% CI: 8.7%–63.0%) between groups (100% in patients with acute HP vs. 64% in the chronic stage). These results remained the same after adjustment to the age of 60. Fig. 4 shows the expected and observed survival curves according to disease stage at diagnosis. Survival analysis stratified according to whether HRCT findings met the criteria for UIP22 showed no significant differences in the 10-year survival between groups, either in the entire study sample (16.3%; 95% CI: 0.0%–64.4%), or in patients with chronic HP without criteria for UIP (16.8%; 95% CI: 0.0%–74.2%) (Fig. 5).
This is the first prospective study, and the largest study to date, of patients with HP attributable to exposure to concealed feathers at home, diagnosed over a 10-year period. Chronic HP was the most common form of presentation (67%). We stress that 10 (45%) patients with this chronic HP presented clinical features of UIP, and IPF was initially suspected. These results correlate with those of our previous study undertaken to re-evaluate patients previously diagnosed with IPF, which showed that 43% of patients with IPF were in fact CHP,6 and that 10/16 (63%) of these IPF patients exposed to feather bedding had CHP whereas “only” 10/30 (33%) of IPF patients not exposed had CHP.6 Both studies reinforce the need for a prompt detailed history of environmental exposure in IPF patients, which should include questions regarding exposure to feather bedding.22
The etiology of HP in the various series reported is unknown in 25%–60% of cases.10,13,14 In-depth assessment of the clinical history and the use of tests such as those used in the present study are needed to determine the etiology. Until recently, in routine clinical practice concealed feathers at home were not usually considered as a cause of HP, and so in some cases in which the etiology of HP remains unknown the disease may have been secondary to this exposure. Indeed, in the present series of 127 patients diagnosed with HP, feather exposure was identified as the cause in 26% of patients, a rate that is not so different from those reported in series without an etiological diagnosis.10,13,14
The present calculated prevalence of HP due to feather duvets and pillows is 6.2 out of 100 000 feather bedding users over a 10-year period. In fact, this prevalence is much lower than that of BBD (54.6/100 000, or roughly 5/10 000 bird breeders), although these prevalence rates differ less when considering the absolute numbers (1.9/100 000 inhabitants for BBD vs. 4.9/100 000 inhabitants for feather bedding HP).
Authors’ opinions differ as to whether the diagnostic performance of ssIgG detection and SIC have been validated. According to data from our center,3 the sensitivity and specificity of ssIgG determination by ELISA in the diagnosis of BBD are 84.2% [95% CI: 62.4–94.5] and 22% [95% CI: 12%–36.7%], respectively.3 In relation to the SIC, a recent review reported a sensitivity of 85% and specificity of 86.2% for diagnosis of HP to avian antigens and fungus,7 figures similar to those obtained by other authors.4,8,23 Thus, a positive test result may be taken to indicate a high probable diagnosis of HP.5 Nevertheless, patients with chronic fibrosis tend to have lower inflammatory cell infiltrates, indicating perhaps that the test may have less sensitivity in this patient subgroup.5 Further studies are need to determine the diagnostic yield of SIC in this fibrotic HP group.
The survival of patients with acute HP over the 10-year study period was 100% compared to only 64% in patients with chronic HP. Adjusted analyses and expected survival curves for the general population indicate that these results are unlikely to be due to the differences in age between groups. This deterioration in patients with chronic HP was observed despite the fact that they avoided further contact with concealed feathers and received treatment with oral steroids. This poor prognosis of chronic HP in relation to the overall follow-up of our series of CHP.24 and other series referred to in Appendix in Table 4, suggests that physicians should consider exposure to concealed feathers in order to allow early identification of this etiology. On the other hand, there were no differences in the survival of patients with chronic HP with a HRCT with UIP pattern and those without a UIP pattern, although the small number of patients studied may have influenced these results.
In the lung biopsy some patients clinically classified as having “acute” HP presented some degree of fibrosis, considered a pathological feature of chronic HP.9 This discordance is likely due to the arbitrary definition of acute and chronic HP, based on symptom duration. In biological terms, the apparent discordance between the clinical acute form and some pathologic characteristics of CHP is due to the fact that fibrosis is not a histologic lesion of sudden onset; rather, the histological features of inflammation and fibrosis are a continuum of lesions. The presence of clubbing in nearly half of the patients (45%) with acute HP, the presence of velcro crackles in 36%, and the observation of peripheral microcysts in 27% of these acute cases in the HRCT should be interpreted in the same way. In fact, in the initial historical description of the pathology of HP in Farmer's lung, Barrowcliff and Arblaster stated that: “Thus fibrosis, a marked feature of the chronic disease and always present to some degree in those cases described as being acute….”.25
The manufacturing process of European feather bedding companies is rigorous, with thorough washing and drying of the feathers used for comforters, blankets, pillows and duvets (personal communication with three main Spanish manufacturers). In Spain, feathers are usually purchased from goose slaughterhouses where foie gras is made, although some companies import feathers from other countries. Further studies are needed to elucidate whether the impact of feather bedding on HP development varies according to its origin.
Our study has several limitations: (1) it is a single-center study performed in one area of the city; (2) the focus of the evaluation was occult avian antigens in feather bedding from different commercial sources; (3) the possible contamination from environmental fungi during the recollection of the feathers by patients cannot be absolutely ruled out; (4) some patients were confirmed by a positive SIC result; although the SIC has been validated as a diagnostic procedure in some studies4,7,8,23 and has been routinely performed as a diagnostic tool at our center for four decades,26 it is still to be accepted as a validated test by some experts in ILD.
Although it is unnecessary to establish the diagnosis in all patients with suspected HP by histopathology,22,27 in the present series the diagnosis was in fact confirmed by characteristic histopathological features of HP in over 50% of patients (17/33). The need for evidence-based or consensus-based clinical practice guidelines for diagnosis of HP is clear, and indeed is long overdue.
In conclusion, in this prospective series of ILD patients, HP was attributed to occult avian or fungal, antigens present in feather duvets or pillows in 26% of the patients diagnosed with HP. The prognosis of chronic HP following this exposure is poor, with a 10-year survival rate after symptom onset of 64%. The diagnostic protocol used in this prospective study may help to identify occult environmental factors such as the etiology of disease in patients presenting with ILD/interstitial pneumonia of unknown origin, including those patients presenting with a UIP pattern, and may help to establish the diagnosis of chronic HP in patients initially diagnosed with IPF.
FundingThe present study has been given support by: Grant FIS PI13/01377 from the Instituto de Salud Carlos III cofinanced by the European Regional Development FUND (FEDER), Grant of Cellex Fundation and Grant of Sociedad Española de Neumología (SEPAR) 2013. The sponsors of the study played no role in the study's design, conduct, and reporting.
Conflict of InterestThe authors declare no conflict of interest.
The authors wish to thank Rosa Llòria for her help in the edition of this manuscript.