(i) Analyze the effect of altitude above the sea level on the mortality rate in patients undergoing invasive mechanical ventilation. (ii) Validate the traditional equation for adjusting PaO2/FiO2 according to the altitude.
DesignA prospective, observational, multicenter and international study conducted during August 2016.
PatientsInclusion criteria: (i) age between 18 and 90 years old, (ii) admitted to intensive care unit (ICU) situated at the same altitude above the sea level (AASL) in which the patients has stayed, at least, during the previous 40 days and (iii) received invasive MV for at least 12h.
Material and methodsAll variables were registered the day of intubation (day 0). Patients were followed until death, ICU discharge or day 28. PaO2/FiO2 ratio was adjusted by the AASL according to: PaO2/FiO2*(barometric pressure/760). Categorical variables were compared with χ2 and Cochran–Mantel–Haenszel test. Continuous variables with Mann–Whitney. Correlation between continuous variables was analyzed graphically and analytically. Logistic regression model was constructed to identify factors associated to mortality. Kapplan–Meier method was used to estimate the probability of survival according to the altitude. A 2-side p value <0.05 was consider significant.
Results249 patients (<1500m n=55; 1500 to <2500m n=20; 2500 to <3500m n=155 and ≥3500m n=19) were included. Adjusted and non-adjusted PaO2/FiO2 were correlated with several respiratory and non respiratory variables. None discordances between non adjusted and adjusted PaO2/FiO2 were identified. However, several correlations were appreciated only in patients situated <1500m or in >1500m. Seventy-nine patients died during the ICU stayed (32%). The mortality curve was not affected by the altitude above the sea level. Variables independently associated to mortality are: PEEP, age, systolic arterial blood pressure, and platelet count. AUROC: 0.72.
ConclusionIn acclimatized patients undergoing invasive mechanical ventilation, the traditional equation for adjusting PaO2/FiO2 according the elevation above the sea level seems to be inaccurate and the altitude above the sea level does not affect the mortality risk.
1) Analizar el efecto de la altitud por encima del nivel del mar en la tasa de mortalidad de pacientes sometidos a ventilación mecánica invasiva, y 2) Validar la ecuación tradicional de ajuste de PaO2/FiO2, de acuerdo con la altitud.
DiseñoEstudio internacional prospectivo, observacional y multicéntrico realizado durante agosto de 2016.
PacientesCriterios de inclusión: 1 Edad comprendida entre 18 y 90 años, 2 Haber sido ingresado en una unidad de cuidados intensivos (UCI) situada a la misma altitud por encima del nivel del mar (AASL) en la cual el paciente haya estado durante al menos los 40 días previos al estudio, y 3) Haber recibido ventilación mecánica (VM) durante al menos 12h.
Materiales y métodosTodas las variables se registraron el día de la intubación (día 0). El seguimiento se realizó hasta la muerte del paciente, el alta de la UCI o el día 28. El cociente PaO2/FiO2 se ajustó según los criterios de la AASL de acuerdo con: PaO2/FiO2*(presión barométrica/760). Las variables categóricas se compararon mediante la prueba de χ2 y el test Cochran-Mantel-Haenszel, y las variables continuas con el test de Mann–Whitney. La correlación entre las variables continuas se analizó de forma gráfica y analítica. Para identificar los factores asociados a la mortalidad se elaboró un modelo de regresión logística. Se utilizó el método de Kaplan-Meier para estimar la probabilidad de supervivencia de acuerdo con la altitud. Un valor de p<0,05 en la prueba bilateral se consideró como significativo.
ResultadosSe incluyeron 249 pacientes (<1.500m, n=55; 1.500 a <2.500m, n=20; 2.500 a <3.500m, n=155 y ≥3.500m, n=19). El cociente PaO2/FiO2 mostró correlación con las variables graves tanto respiratorias como no respiratorias. No se registraron discordancias entre el cociente PaO2/FiO2 ajustado y sin ajustar. Únicamente se observaron diversas correlaciones entre los pacientes situados a <1.500m o a >1.500m. Setenta y nueve pacientes (32%) murieron durante la estancia en la UCI. La altitud sobre el nivel del mar no afectó a la curva de mortalidad. Las variables asociadas de forma independiente con la mortalidad fueron la presión positiva al final de la espiración (PEEP), la edad, la presión arterial sistólica y el recuento de plaquetas. El área bajo la curva ROC (AUROC) fue de 0,72.
ConclusiónEn pacientes aclimatados sometidos a ventilación mecánica invasiva la ecuación tradicional para ajustar el cociente PaO2/FiO2, de acuerdo con la elevación sobre el nivel del mar parece inexacta. Por otro lado, la altitud por encima del nivel del mar no afecta al riego de mortalidad.
Geographical areas above the sea level are associated to adverse conditions for the Life. For example, although the oxygen concentration remains constant with the trophosphere, the barometric pressure falls, which determines a condition called hypobaric hypoxemia. Indeed, the humidity and temperature, factors that may contribute to airway reactivity, insensible water losses, ventilatory changes and alterations in pulmonary hemodynamics,1 also decrease in these areas. Fast ascend to elevated areas is associated to well know entities such us mountain sickness, High Altitude Pulmonary Edema (HAPE), High Altitude Cerebral Edema (HACE) and cardiovascular collapse.2 However, those who stay for a long period of time above the sea level develop mechanisms to compensate this adverse environment; therefore previously mentioned entities are unusual. Acclimatization to altitude is a term use to describe physiological changes that occur over several weeks with the aim to increase the endurance against the deleterious effect of staying above the sea level.3–5
Invasive mechanical ventilation is the cornerstone intervention for supporting severe respiratory failure. Unfortunately, despite 385 million people permanently residing at elevations above 1500m6 and adverse effects of chronic exposure to high altitude are well demonstrated3,5,7; the knowledge regarding the effect of altitude above the sea level in acclimatized patients under invasive mechanical ventilation is scarce. Furthermore, several worldwide surveys regarding mechanical ventilation have been performed,8–11 but none of them have considered the effect of altitude above the sea level. It is paramount importance to highlight that mechanical ventilators are non pressurized systems and they function at the same atmospheric pressure as the environment. This mean that, despite the patient is under invasive mechanical ventilation, their alveolar pressure and their arterial pressure is lower than at sea level for the same inspiratory pressure.
On the other hand, with the aim to maintain the biological significance of one variable (e.g. diagnostic criteria for a disease or prognosis factor) sometimes it is necessary to adjust their value according to another variable. For example, it is possible to use the partial oxygen pressure (PaO2) as a surrogate biomarker of the gas exchange if everybody receives the same oxygen inspiratory fraction (FiO2). However, as it is possible to prescribe different FiO2, it is common to relativize the PaO2 value according to the FiO2 (PaO2/FiO2). Partial oxygen pressure is also dramatically influenced by the atmospheric pressure, thus in high altitudes above the sea level the biological significance of a specific PaO2/FiO2 could differ than at sea level. A mathematical equation has been proposed to adjust the PaO2/FiO2 according to the altitude (PaO2/FiO2*[barometric pressure/760]).12,13 However, as far as we know, it is an empiric equation and none have validate it.
Studying the effect of the altitude above the sea level is not merely an academic exercise as, if the outcome of lowlanders differs from highlanders, it may imply that one group are exposed to different risks and require special interventions than the other. Indeed, mechanisms for adaptation to hypobaric hypoxia could be useful in other hypoxemic diseases such as chronic obstructive pulmonary diseases (COPD), myocardial ischemia or ARDS.3,12,14–16 On the other hand, validating the equation for adjusting the PaO2/FiO2 according to the altitude is also of paramount importance as is the variable used to define, stratify and select the best treatment for patients with ARDS.12,17–19 Likewise, due to the fact that PaO2/FiO2 is associated to the outcome in general population under invasive mechanical ventilation, predicting their outcome would be inaccurate in patients living in high altitudes.10,20
We hypothesize that altitude above the sea level affects the outcome of acclimatized patients undergoing invasively mechanically ventilated.
The aim of this study is (i) to validate the traditional equation for adjusting PaO2/FiO2 according to the altitude and (ii) to analyze the effect of altitude above the sea level on the mortality rate in patients undergoing invasive mechanical ventilation.
Material and methodDesign: prospective, observational, multicenter and international study conducted during August 2016.
Inclusion criteria: age between 18 and 90 years old, admitted to intensive care unit (ICU) situated at the same altitude above the sea level in which the patient has stayed, at least, during the previous 40 days before ICU admission and received invasive MV for at least 12h. The full list of centers can be appreciated in supplementary material. Two hospitals (“Obrero N°1” Hospital, Bolivia and Donostia University Hospital, Spain) retrieved the data retrospectively. Exclusion criteria include: home oxygen therapy, metastatic cancer, pregnancy, and absence of informed consent.
ProtocolGeneral and national coordinators recruited local researchers from eligible ICUs. With the aim of decreasing the bias in practice, only research coordinators directly related to the study were aware of the exact purpose, the timing of the study and variables reported.
A website was developed to register demographic characteristics (age, weight and height), comorbidities (COPD, diabetes, chronic heart failure, chronic renal failure, active solid cancer and active hemato-oncologic cancer), clinical data (arterial systolic pressure and APACHE II score), ventilator parameters (tidal volume, peak pressure, plateau pressure, positive end expiratory and respiratory rate) and analytical (arterial blood gases, hemoglobin, leukocytes, platelets, C reactive protein, creatinine and total bilirubin) of patients on the day of starting invasive mechanical ventilation.
The PaO2/FiO2 was recorded and then, in patients treated 1000m above the sea level, adjusted according to following equation: [PaO2/FiO2*(barometric pressure/760)].12,13 Thus, two PaO2/FiO2 were contemplated: non-adjusted and adjusted.
According to Zubieta-Calleja et al.,21 acclimation to a specific altitude was defined if the patient permanently stayed during 40 days at the same altitude above sea level. Altitude was expressed in meters above the sea level. Severe Sepsis and septic shock were defined according to the American College of Chest Physicians/Society of Critical Care Medicine22 as it was the current definition at the time that the study was performed. Active haemato-oncologic or active solid cancer was defined if the patients were diagnosed with cancer (or metastasis), received chemotherapy, radiotherapy or biological therapy during the 60 days before the enrollment.
The study was registered at the Clinical trial website (NCT02871063), was approved by the ethics committee of the Universidad “El Bosque” – Bogota – Colombia and, when required, by the institutional review boards at the individual sites.
Quality controlWith the aim of decreasing errors during data entry on the website, a range of continuous variables and codification for qualitative variables was set up. Likewise, once the recruiting period finished, 10% of the patients were randomly selected for a second review by the general coordinators of the study (MJ, GO, PC). Incomplete records were not considered.
Statistical analysisAll clinical and analytical variables correspond to the day of oral intubation and initiating mechanical ventilation. Continuous variables were expressed as the median with interquartile range and compared using Mann–Whitney test. Categorical variables were expressed as the absolute frequency with proportions and were compared using the χ2 test. The strength of the association was expressed as the odds ratio (OR) and its 95% confidence interval (CI).
With the aim to validate the equation for adjusting the PaO2/FiO2, the correlation adjusted and non adjusted PaO2/FiO2 and other continuous variables were compared in patients situated below and above 1500m of altitude. Secondly, both PaO2/FiO2 were stratified according to criteria used by Esteban et al.10 in: <100; 100–149; 150–199; 200–300 and >300 and then the mortality rate distribution between <1500m and >=1500m was compared using Cochran–Mantel–Haenszel test.
With the aim to identify the association between altitude and mortality, a logistic regression model was performed. The maximum model included all variables with a p value <0.1 in the univariate analysis. Then, one by one the variables with the highest p value were removed until all variables in the model had a p value <0.05 (backward step procedure). The AUROC, sensitivity, specificity, positive and negative likelihood ratio were calculated. The Kapplan–Meier method was used to estimate the probability of survival according to altitude categorized in <1500; 1500–2500; 2500–3500 and >3500.
A 2-sided p value less than 0.05 was considered statistically significant. All the analyses were performed using R package.23
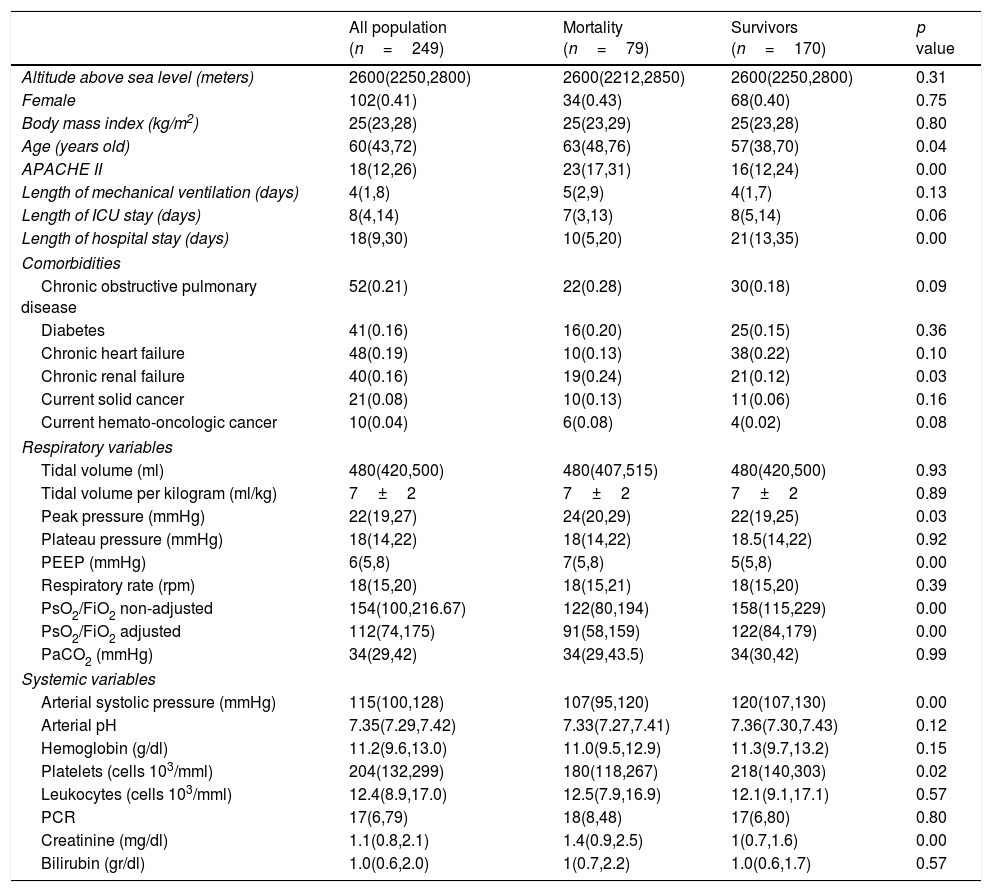
ResultsThirty medical-surgical ICUs with 249 patients from 7 countries (Bolivia, Brazil, Colombia, Ecuador, Mexico, Peru and Spain) were analyzed (Table S1). Patients were assisted at the following altitudes: <1500m n=55; 1500–2500m n=20; 2500–3500m n=155 and >3500m n=19. Reason for ICU admission was: pulmonary sepsis n=111; extrapulmonary sepsis n=42; trauma n=16; acute respiratory failure n=14; stroke n=8; post-operative n=6; acute heart failure n=4; burned n=3, cardiac arrest n=3, hemorrhagic shock n=3, pancreatitis n=3 and miscellaneous n=36. Characteristics of all patients can be appreciated in Table 1. Comparison between highlanders and lownlanders can be appreciated in supplementary Table 1.
Univariate and multivariate analysis for mortality.
| All population (n=249) | Mortality (n=79) | Survivors (n=170) | p value | |
|---|---|---|---|---|
| Altitude above sea level (meters) | 2600(2250,2800) | 2600(2212,2850) | 2600(2250,2800) | 0.31 |
| Female | 102(0.41) | 34(0.43) | 68(0.40) | 0.75 |
| Body mass index (kg/m2) | 25(23,28) | 25(23,29) | 25(23,28) | 0.80 |
| Age (years old) | 60(43,72) | 63(48,76) | 57(38,70) | 0.04 |
| APACHE II | 18(12,26) | 23(17,31) | 16(12,24) | 0.00 |
| Length of mechanical ventilation (days) | 4(1,8) | 5(2,9) | 4(1,7) | 0.13 |
| Length of ICU stay (days) | 8(4,14) | 7(3,13) | 8(5,14) | 0.06 |
| Length of hospital stay (days) | 18(9,30) | 10(5,20) | 21(13,35) | 0.00 |
| Comorbidities | ||||
| Chronic obstructive pulmonary disease | 52(0.21) | 22(0.28) | 30(0.18) | 0.09 |
| Diabetes | 41(0.16) | 16(0.20) | 25(0.15) | 0.36 |
| Chronic heart failure | 48(0.19) | 10(0.13) | 38(0.22) | 0.10 |
| Chronic renal failure | 40(0.16) | 19(0.24) | 21(0.12) | 0.03 |
| Current solid cancer | 21(0.08) | 10(0.13) | 11(0.06) | 0.16 |
| Current hemato-oncologic cancer | 10(0.04) | 6(0.08) | 4(0.02) | 0.08 |
| Respiratory variables | ||||
| Tidal volume (ml) | 480(420,500) | 480(407,515) | 480(420,500) | 0.93 |
| Tidal volume per kilogram (ml/kg) | 7±2 | 7±2 | 7±2 | 0.89 |
| Peak pressure (mmHg) | 22(19,27) | 24(20,29) | 22(19,25) | 0.03 |
| Plateau pressure (mmHg) | 18(14,22) | 18(14,22) | 18.5(14,22) | 0.92 |
| PEEP (mmHg) | 6(5,8) | 7(5,8) | 5(5,8) | 0.00 |
| Respiratory rate (rpm) | 18(15,20) | 18(15,21) | 18(15,20) | 0.39 |
| PsO2/FiO2 non-adjusted | 154(100,216.67) | 122(80,194) | 158(115,229) | 0.00 |
| PsO2/FiO2 adjusted | 112(74,175) | 91(58,159) | 122(84,179) | 0.00 |
| PaCO2 (mmHg) | 34(29,42) | 34(29,43.5) | 34(30,42) | 0.99 |
| Systemic variables | ||||
| Arterial systolic pressure (mmHg) | 115(100,128) | 107(95,120) | 120(107,130) | 0.00 |
| Arterial pH | 7.35(7.29,7.42) | 7.33(7.27,7.41) | 7.36(7.30,7.43) | 0.12 |
| Hemoglobin (g/dl) | 11.2(9.6,13.0) | 11.0(9.5,12.9) | 11.3(9.7,13.2) | 0.15 |
| Platelets (cells 103/mml) | 204(132,299) | 180(118,267) | 218(140,303) | 0.02 |
| Leukocytes (cells 103/mml) | 12.4(8.9,17.0) | 12.5(7.9,16.9) | 12.1(9.1,17.1) | 0.57 |
| PCR | 17(6,79) | 18(8,48) | 17(6,80) | 0.80 |
| Creatinine (mg/dl) | 1.1(0.8,2.1) | 1.4(0.9,2.5) | 1(0.7,1.6) | 0.00 |
| Bilirubin (gr/dl) | 1.0(0.6,2.0) | 1(0.7,2.2) | 1.0(0.6,1.7) | 0.57 |
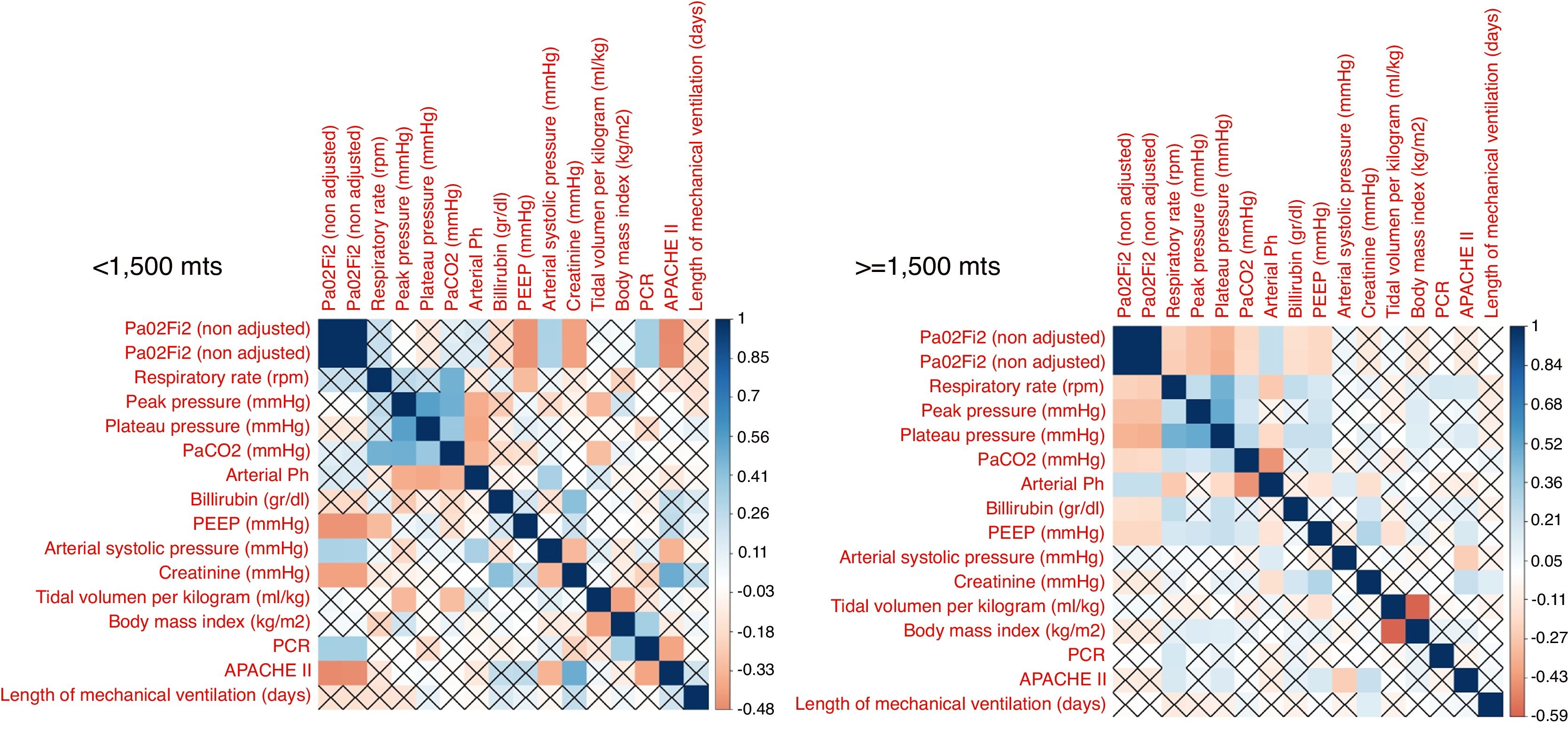
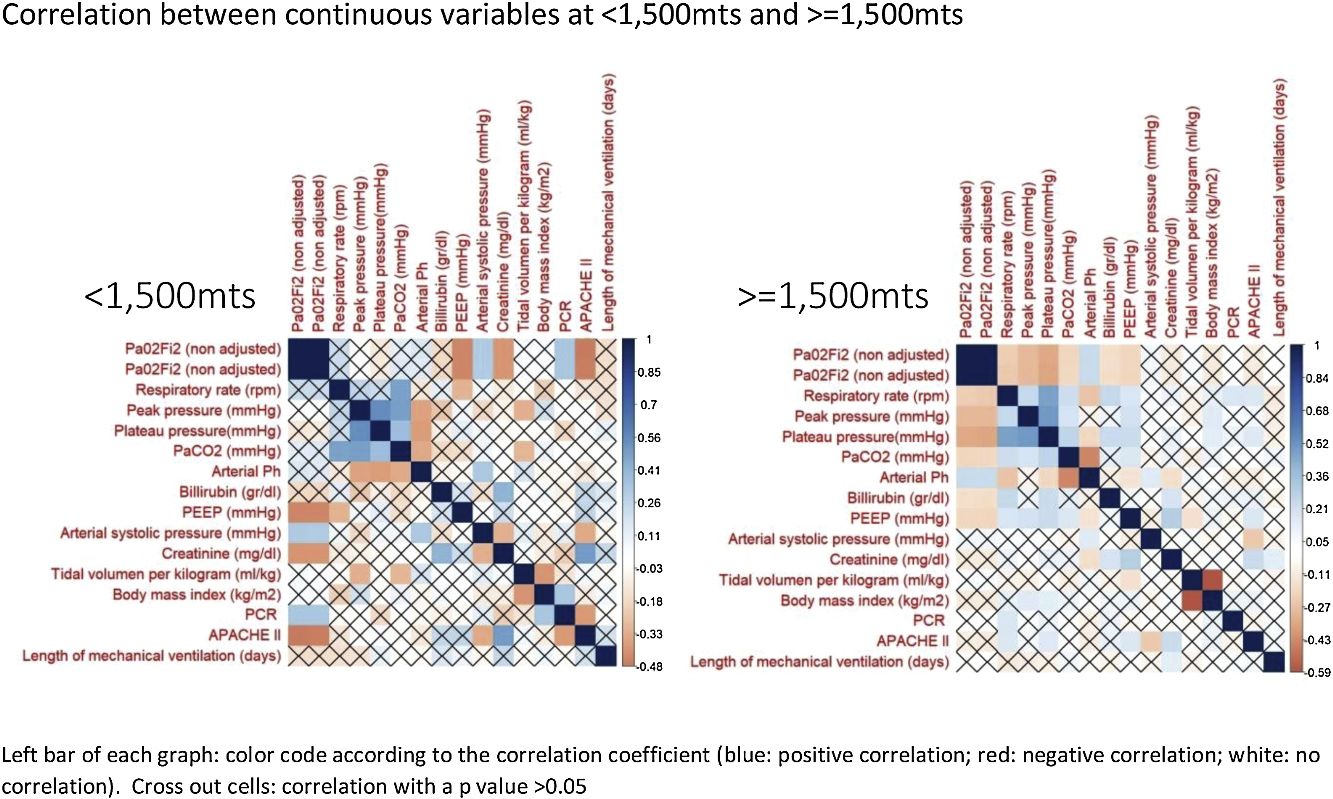
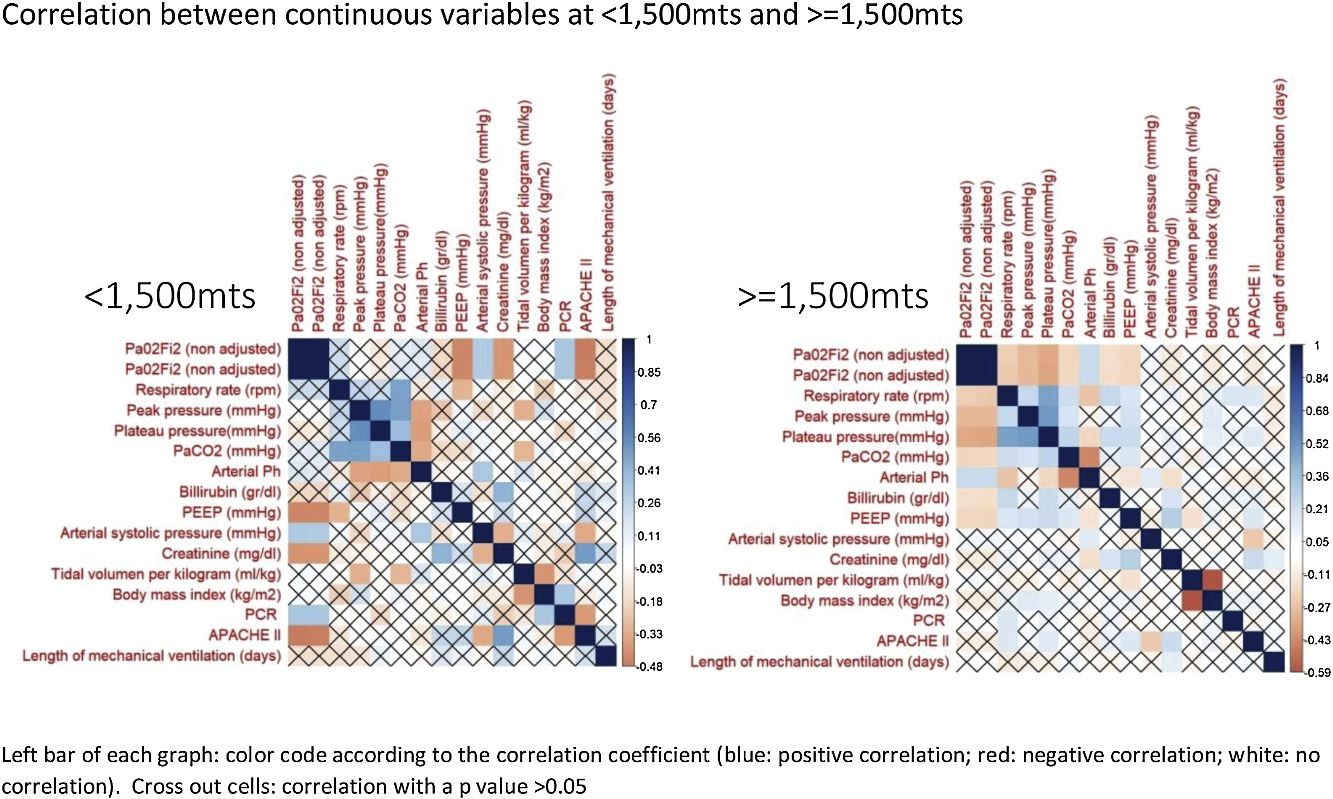
The correlation between all continuous variables, including PaO2/FiO2 (non adjusted) and PaO2/FiO2 (adjusted), is shown in Fig. 1 and supplementary Fig. 1. Adjusted and non-adjusted PaO2/FiO2 were correlated with PEEP, arterial systolic blood pressure and creatinine concentration below and above 1500m. However, respiratory rate, peak pressure, plateau pressure, PaCO2, arterial pH, tidal volume per kilogram, length of mechanical ventilation, body mass index and bilirubin concentration were correlated with both PaO2/FiO2 only in high altitude patients. PCR concentration and APACHE II score were correlated with both PaO2/FiO2 at sea level but not in altitude patients. None discordances between non adjusted and adjusted PaO2/FiO2 were identified.
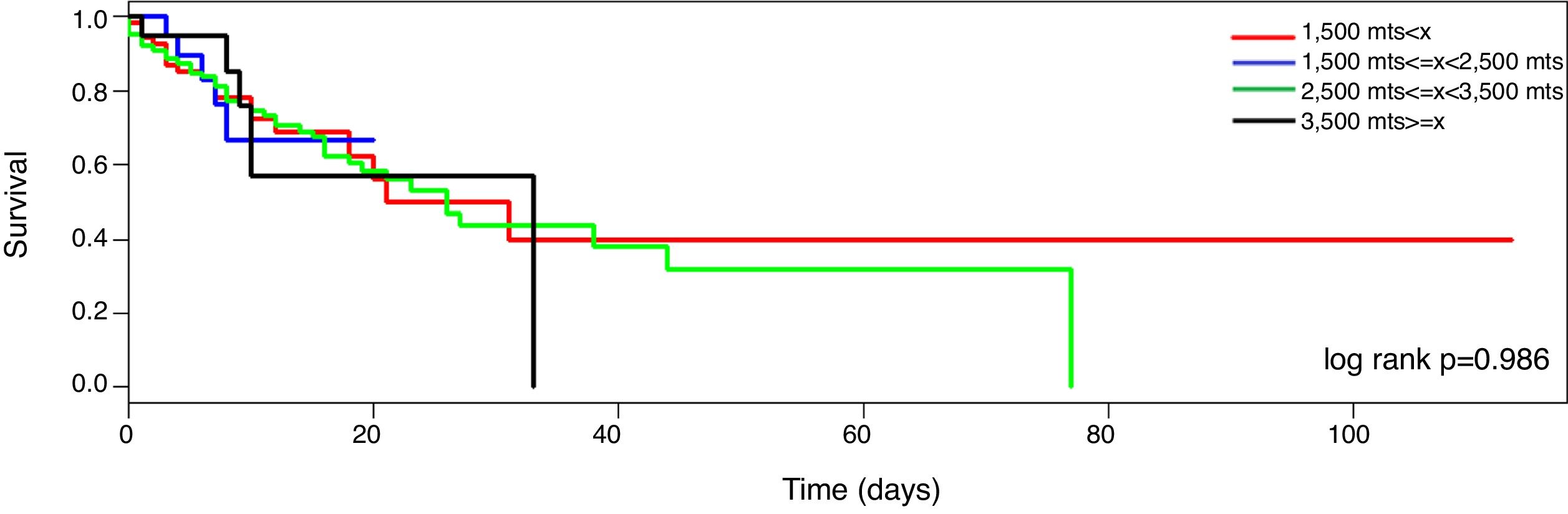
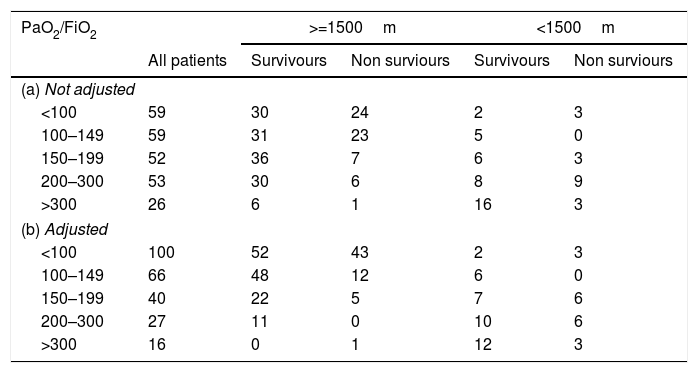
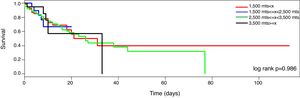
MortalitySeventy-nine patients died during the ICU stayed (32%). The mortality curve was not affected by the altitude above the sea level (Fig. 2, log-rank 0.986). However, when the mortality is stratified according to the PaO2/FiO2 (non adjusted and adjusted) and the altitude dichotomized in the two main groups (<1500m and >=1500m), the observer mortality in each PaO2/FiO2 range was different between both altitudes (Table 2).
Cochran–Mantel–Haenszel test (PaO2/FiO2 and mortality stratified according to altitude above sea level).
| PaO2/FiO2 | >=1500m | <1500m | |||
|---|---|---|---|---|---|
| All patients | Survivours | Non surviours | Survivours | Non surviours | |
| (a) Not adjusted | |||||
| <100 | 59 | 30 | 24 | 2 | 3 |
| 100–149 | 59 | 31 | 23 | 5 | 0 |
| 150–199 | 52 | 36 | 7 | 6 | 3 |
| 200–300 | 53 | 30 | 6 | 8 | 9 |
| >300 | 26 | 6 | 1 | 16 | 3 |
| (b) Adjusted | |||||
| <100 | 100 | 52 | 43 | 2 | 3 |
| 100–149 | 66 | 48 | 12 | 6 | 0 |
| 150–199 | 40 | 22 | 5 | 7 | 6 |
| 200–300 | 27 | 11 | 0 | 10 | 6 |
| >300 | 16 | 0 | 1 | 12 | 3 |
(a) Not adjusted PaO2/FiO2
Cochran–Mantel–Haenszel M^2=16.428, df=4, p-value=0.002495.
(b) Adjusted PaO2/FiO2
Cochran–Mantel–Haenszel M^2=19.097, df=4, p-value=0.0007523.
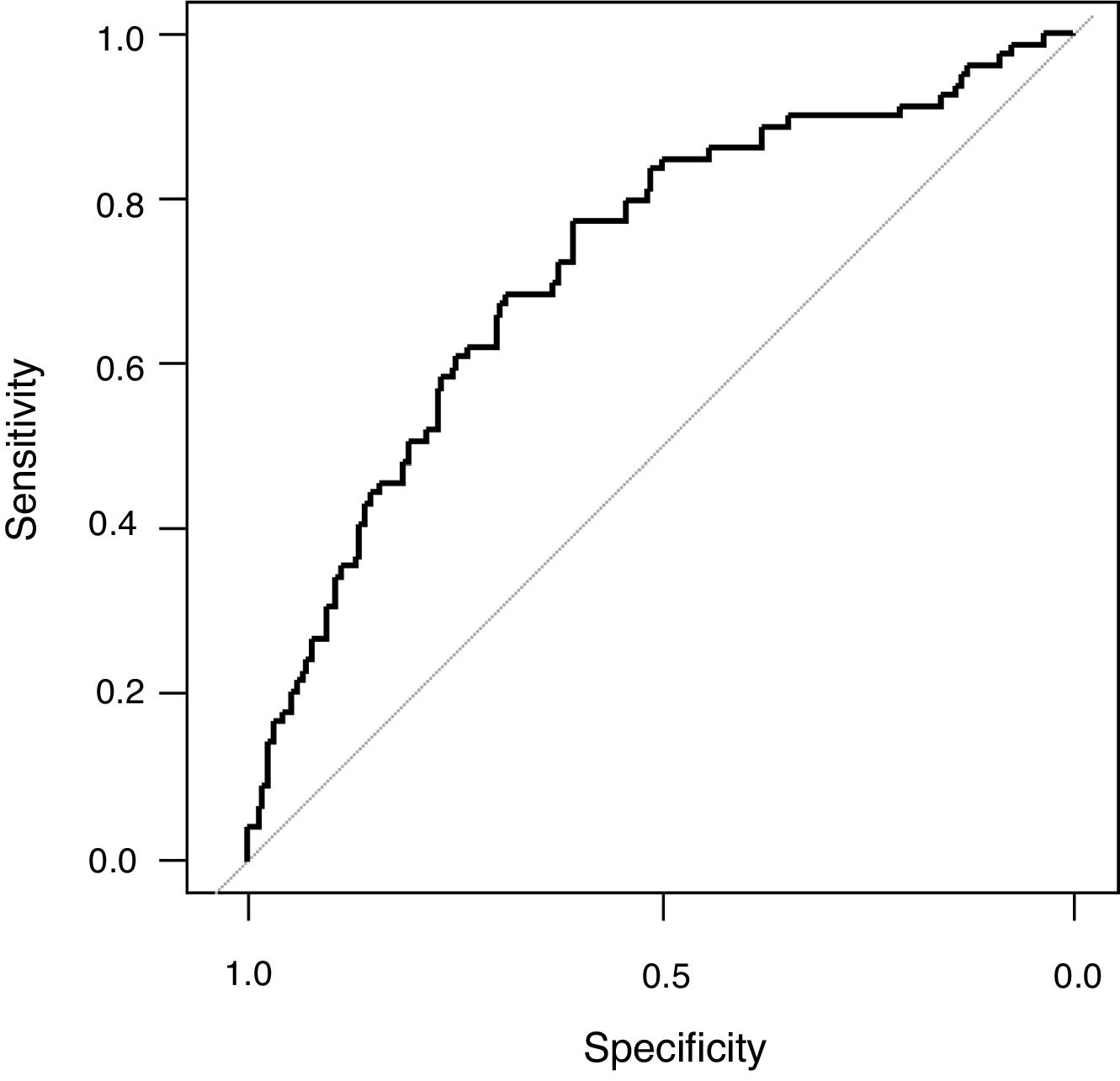
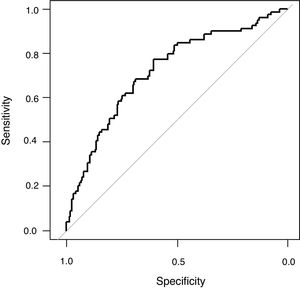
The univariate analysis demonstrated that age, haemato-oncology disease, COPD, chronic renal failure, creatinine, platelets count, arterial systolic blood pressure, APACHE II, PaO2/FiO2 (non-adjusted), PaO2/FiO2 (adjusted), peak pressure and PEEP were associated to mortality (Table 1). All these variables except PaO2/FiO2 (non-adjusted) and APACHE II were included in the maximum logistic regression model. Finally, PEEP (OR 1.17; 95% CI 1.06; 1.31; p: <0.01), age (OR 1.02; 95% CI 1.01; 1.04; p: <0.01), systolic arterial blood pressure (OR 0.98; 95% CI 0.96; 0.99; p: <0.01) and platelet count (OR 0.9999974; 95% IC 0.9999949; 0.9999997, p: 0.03) were independently associated to mortality (Table 1). The AUROC of the logistic model was 0.72 (95% CI: 0.65; 0.79), the sensibility 0.27 (0.17; 0.38), specificity 0.91 (0.85; 0.95), positive likelihood ratio 2.82 (1.56; 5.11) and negative likelihood ratio 0.81 (0.70; 0.93) (Fig. 3). The final regression model did no change when PaO2/FiO2 (non-adjusted) was substituted by PaO2/FiO2 (adjusted).
DiscussionMain results of this pioneer study are: (i) the recommended formula for adjusting the PaO2/FiO2 ratio according to the altitude above the sea level seems to be inaccurate and (ii) the altitude above the sea level not influence the mortality risk.
According to our results, in term of mortality prediction or correlation with physiological variables, the traditional equation for adjusting the PaO2/FiO2 according to the altitude above the sea level does not add any advantage over the raw variable. This fact was expected, as the equation that links both variables is lineal. However, it is important to highlight that some correlations identified in patients at <1500m were not observed in patients at >=1500m and visceversa. Furthermore, the mortality rate, stratified according to PaO2/FiO2, also differ in both groups of patients. We hypothesize that these finding evidence that it would be necessary to adjust the PaO2/FiO2 according to the altitude; but the current equation is not accurate. Future studies with more patients and altitude levels should clarify this issue.
On the other hand, one of the hallmark studies performed in non-specific population under invasive mechanical ventilation was conducted by Esteban et al.10 It include 5183 patients from 361 ICU of Europe, North and Latin America and reported that several variable including PaO2/FiO2 <200 are independently associated to mortality.10 Likewise, Sudarsanam et al.20 analyzed 200 consecutive patients admitted to an medical UCI and reported that type 1 respiratory failure is also independent predictor of mortality. On line with them, we reported that PEEP, age, systolic blood pressure and platelet count were associated to mortality risk. However, in our study neither in the regression model nor in the survival curve the mortality rate was affected by the altitude above the sea level. This result, that should be validated in future studies, rejected the hypothesis of this study and could reflect the efficacy of acclimatization mechanisms to altitude. Based on this result, we speculate that people acclimatized to high altitude have physiologic mechanisms to compensate the deleterious effect that high altitude bear. Considering this, one natural question arises: “At sea level, Is the outcome of patients adapted to high altitude similar to lowlanders?”. Exploring the differences between humans exposed and not exposed to hypobaric hypoxia has the potential to identify mechanisms important in critical illness and perhaps to alter our therapeutic focus toward increasing the efficiency of oxygen utilization rather than improving delivery.24
We have to accept that this study has some limitations. Firstly, the number of patients in extreme altitude is very small which may be explained by the low number of ICUs at those geographical areas. This may influence the result because this subgroup of patients and the clinical practice in these ICUs could differ from those at sea level. Likewise, it could be possible that specific subgroups of patients (e.g. ARDS, surgical, etc.) carried specific features that were not identify in this study. Secondly, despite enrolling a large number of ICUs from South America and Spain, our sample does not include all possible altitudes. Thirdly, the study was conducted for one month and may not represent year-round. Fourth, two centers collected the data retrospectively. Although this is an evident protocol deviation, it is very improbable that may influence the results as booth ICUs have an informatics medical records that allow them to include patients and retrieve their data. Fifth, the mechanical ventilation between different hospitals was not standardized. This limitation is common to all observational study. Sixty, recently new variables such as driving pressure and mechanical power have demonstrated a close relation with the outcome. Future studies will have to address the effect altitude above the sea level on these variable. Finally, as this is a pioneer study, the knowledge previously available to interpret its result could be insufficient or inaccurate. Contrarily, this study has several strengths. Firstly, we used an objectively and previous published definition for considering a patient adapted to a specific altitude.21 Unfortunately, analytical or genetic biomarkers for defining adaptation to a specific altitude are not available or are inaccurate. Secondly, the outcome we have address (mortality) is objective. Thirdly, we included several procedures (e.g., website, quality-control, etc.) to reduce the risk of bias. Likewise, the proportion of patient excluded from the final analysis, as their data were incomplete reflect the strict of methodology we applied.
ConclusionsIn acclimatized patients undergoing invasive mechanical ventilation, the traditional equation for adjusting PaO2/FiO2 according the elevation above the sea level seems to be inaccurate and the altitude above the sea level does not affect the mortality risk.
FundingThis study was partially supported by Escuela de Medicina de la Universidad Internacional del Ecuador (UIDE).
Authors’ contributionsPC, GO, MJ, participated in the design of the study and performed the statistical analysis. PC, GO, MJ, MG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. PC, GO, MJ, MG, FG, FM, JM, JV, OB, SS, FV, FM, JT, CI BV, FZ, AL participate in coordination of their centers and critical care units. All authors read and approved the final manuscript.
Conflict of interestsThe authors declare no conflict of interest.