Tumor mechanobiology is a novel evolving field of research that has already revealed a plethora of knowledge regarding the mechanical aspects of tumors and how these fundamental properties drive tumor development and progression. Intriguingly, understanding the molecular mechanisms of tumor mechanics has allowed us to identify a variety of new targets upon which we can act via the development of mechanotherapeutics. One such target is the Hippo-yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) signaling axis, which forms a key signaling hub associated with tumor mechanobiology in non-small cell lung cancer (NSCLC). Here, we briefly highlight the therapeutic potential of targeting mechanobiology-related pathways in NSCLC, using YAP/TAZ signaling as a representative example.
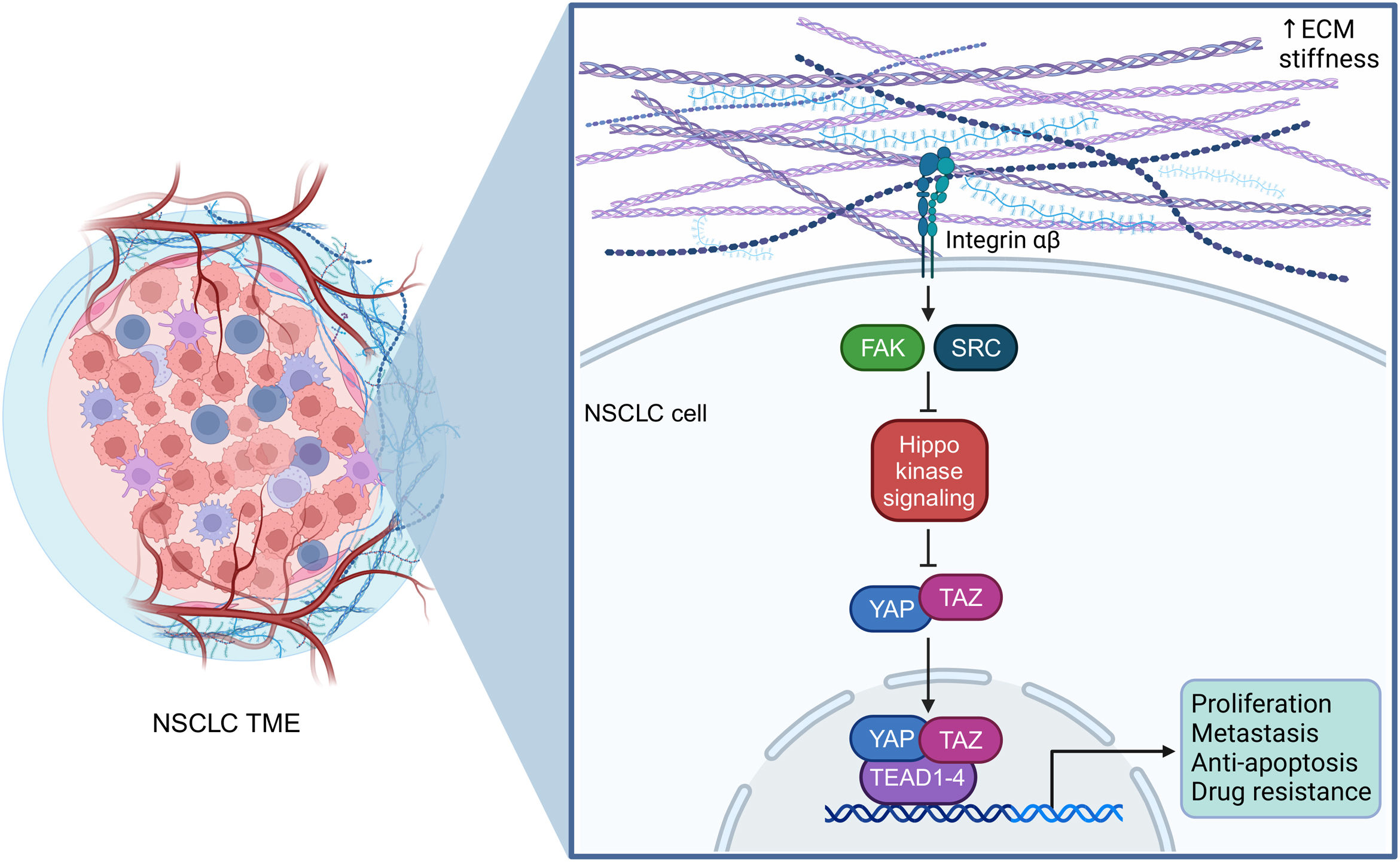
The mechanical properties of NSCLC cells, i.e., (a) their ability to sense extracellular mechanical signals, (b) transduce these cues within their cytoplasm and into their nucleus in the form of oncogenic signaling, and (c) finally respond by eliciting tumor-promoting transcriptional programs that will endow them with malignant characteristics, are essential for their interplay with (i) nearby tumor cells, (ii) the numerous non-tumor cells, and (iii) the extracellular matrix (ECM), all of which comprise the tumor microenvironment (TME). NSCLC cells reside within this complex and dynamic niche hijacking its components and creating a supportive network that will allow them to continuously flourish. The ECM, composed of fibronectin, fibrillar collagen, laminins, elastin, and hyaluronan, represents a large portion of the tumor mass and its stiffness has been shown to impact tumor progression in NSCLC (Fig. 1). On a molecular level, the Hippo-YAP/TAZ signaling axis is the major signaling pathway linked to aberrant mechanotransduction in NSCLC.1 It is an evolutionary conserved pathway that controls cell growth, proliferation, apoptosis, stem cell self-renewal, and is modulated by mechanical cues, the status of cellular energy, and inputs from multiple receptor signaling cascades. YAP and TAZ are key transcriptional co-regulators and the downstream nuclear effectors of the Hippo mechanotransduction pathway, that act by physically binding to members of the transcriptional enhanced associate domain (TEAD) transcription factor family (in humans, there are four TEAD proteins, TEAD1–4) (Fig. 1). Changes in ECM rigidity, due to uncontrolled tumor growth and the production of ECM components by cancer-associated fibroblasts (CAFs), are translated into intracellular biochemical signals that trigger mechano-stimulated tumorigenic effects leading to the activation of YAP/TAZ. In turn, mechano-potentiated YAP/TAZ induce the expression of genes that foster NSCLC cell proliferation, plasticity, invasion, metastasis, angiogenesis, immunosuppression, and drug resistance (Fig. 1). For example, recent data demonstrate that ECM stiffness controls programmed death-ligand 1 (PD-L1) expression via YAP activation, promoting tumor cell proliferation in lung adenocarcinoma (the predominant type of NSCLC).2
YAP/TAZ-TEAD signaling in NSCLC mechanobiology. NSCLC cells reside within a complex and dynamic tumor microenvironment, continuously interacting with nearby tumor cells, non-tumor cells, such as cancer-associated fibroblasts (CAFs) and immune cells, as well as the stiff extracellular matrix (ECM), comprised of collagen fibers, fibronectin, laminins, elastin, and hyaluronan. These physical interactions generate mechanical signals which lead to the intracellular activation of YAP/TAZ-TEAD signaling, promoting the development and progression of NSCLC. ECM, extracellular matrix; FAK, focal adhesion kinase; NSCLC, non-small cell lung cancer; SRC, proto-oncogene tyrosine protein kinase Src; TAZ, transcriptional co-activator with PDZ-binding motif; TEAD, transcriptional enhanced associate domain; TME, tumor microenvironment; YAP, yes-associated protein. This figure was created using the tools provided by BioRender.com.
An important consequence of augmented NSCLC-ECM stiffness that should be considered by cancer researchers and clinicians is that it results in the compression of blood vessels, hence not allowing blood to flow efficiently and hampering oxygen delivery, as well as the access of systematic drugs (targeted therapies, chemotherapeutics, immunotherapy agents) and the infiltration of immune cells. As a proof of concept, losartan, an angiotensin II receptor blocker with a vasodilative effect, has already been used in the clinical setting to decrease accumulating solid stress (i.e., the sum of the physical forces exerted during tumor growth) and enhance drug delivery, such as immune checkpoint inhibitors, in NSCLC.3
Another dimension with clinical relevance concerning YAP/TAZ-TEAD signaling in NSCLC is the fact that its aberrant upregulation promotes resistance to cancer therapy.4 Several resistance mechanisms involving the action of YAP/TAZ-TEAD have been proposed for tyrosine kinase inhibitors (TKIs) that block epidermal growth factor receptor (EGFR), B-Raf proto-oncogene (BRAF), Kirsten rat sarcoma viral oncogene homolog (KRAS), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), for immune checkpoint inhibitors, as well as for chemoradiotherapy.5 All this evidence implies that modulation of the YAP/TAZ-TEAD pathway with small-molecule compounds may ameliorate the efficacy of standard treatment regimens. For instance, in terms of targeted therapy for NSCLC, studies indicate that TEAD inhibition enhances the anti-tumor effect of KRASG12C (the most common KRAS mutation) and EGFR inhibitors in lung adenocarcinoma.6,7
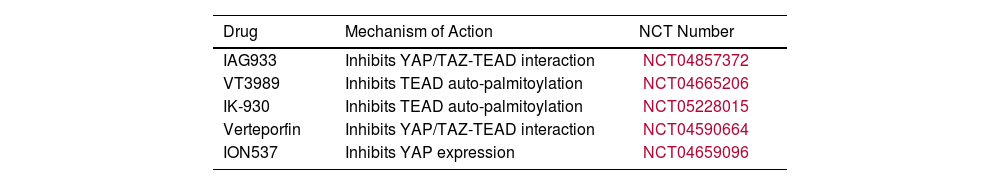
Although YAP and TAZ are regarded as functionally redundant, recent findings suggest that these two paralogs orchestrate distinct gene expression programs, with TAZ chiefly controlling genes associated with the ECM, cancer-cell adhesion, and cancer-cell migration, and YAP mainly controlling genes associated with cancer cell-cycle progression and division.8 Notably, these differential effects on gene transcription are also reflected in the response of NSCLC cells to anti-tumor therapies. Future clinical trials may exploit this “division of labor” between YAP and TAZ through stratifying NSCLC patients based on their YAP or TAZ expression profiles to maximize their response to various treatments. Several clinical trials employing drugs that target YAP/TAZ-TEAD are ongoing and their results are ardently awaited (Table 1).
Clinical Trials Evaluating YAP/TAZ-TEAD-targeting Drugs.
| Drug | Mechanism of Action | NCT Number |
|---|---|---|
| IAG933 | Inhibits YAP/TAZ-TEAD interaction | NCT04857372 |
| VT3989 | Inhibits TEAD auto-palmitoylation | NCT04665206 |
| IK-930 | Inhibits TEAD auto-palmitoylation | NCT05228015 |
| Verteporfin | Inhibits YAP/TAZ-TEAD interaction | NCT04590664 |
| ION537 | Inhibits YAP expression | NCT04659096 |
NCT, National Clinical Trial; TAZ, transcriptional co-activator with PDZ-binding motif; TEAD, transcriptional enhanced associate domain; YAP, yes-associated protein.
The abnormal ECM stiffness exhibited by most tumors may also provide novel imaging biomarkers.9 In this regard, using imaging tools that are able to probe tumor mechanics in vivo, such as magnetic resonance elastography (MRE), will aid clinicians to diagnose NSCLC tumors, perhaps even at an early stage, as well as predict their prognosis and their response to treatment when combined with molecular profiling. Additionally, MRE could be utilized to monitor response to mechanotherapeutics.
In conclusion, mounting evidence implies that aberrant YAP/TAZ-TEAD signaling contributes significantly to NSCLC development and progression. Deciphering the molecular underpinnings of YAP/TAZ-TEAD-mediated mechanosignaling in NSCLC will offer several opportunities for therapeutic targeting and uncover resistance mechanisms to YAP/TAZ-TEAD inhibition. Eventually, the clinical management of NSCLC will include mechanotherapeutics tailored to each individual patient according to the expression of molecular “mechanomarkers”.
FundingNone declared.
Authors’ ContributionsKostas A. Papavassiliou: conceptualization; writing – original draft. Vassiliki A. Gogou: writing – original draft. Athanasios G. Papavassiliou: conceptualization; supervision and review & editing.
Ethical ApprovalNot applicable.
Conflict of InterestsThe authors have no conflict of interests to declare.