In patients with chronic obstructive pulmonary disease (COPD), skeletal muscle dysfunction is a major comorbidity that negatively impacts their exercise capacity and quality of life. In the current guidelines, the most recent literature on the various aspects of COPD muscle dysfunction has been included. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) scale has been used to make evidence-based recommendations on the different features. Compared to a control population, one third of COPD patients exhibited a 25% decline in quadriceps muscle strength, even at early stages of their disease. Although both respiratory and limb muscles are altered, the latter are usually more severely affected. Numerous factors and biological mechanisms are involved in the etiology of COPD muscle dysfunction. Several tests are proposed in order to diagnose and evaluate the degree of muscle dysfunction of both respiratory and limb muscles (peripheral), as well as to identify the patients’ exercise capacity (six-minute walking test and cycloergometry). Currently available therapeutic strategies including the different training modalities and pharmacological and nutritional support are also described.
La disfunción muscular de pacientes con enfermedad pulmonar obstructiva crónica (EPOC) constituye una de las comorbilidades más importantes, con repercusiones negativas en su capacidad de ejercicio y calidad de vida. En la presente normativa se ha resumido la literatura publicada más recientemente sobre los diferentes aspectos del tema y se ha utilizado también la escala Grading of Recommendations Assessment, Development, and Evaluation (GRADE) de recomendaciones sobre el grado de evidencia de las diferentes propuestas de la normativa. Respecto a una población control, se estima que en un tercio de los pacientes EPOC la fuerza del cuádriceps es un 25% inferior incluso en estadios precoces de su enfermedad. Aunque tanto los músculos respiratorios como los de las extremidades están alterados, estos últimos suelen verse mayormente afectados. Diversos factores y mecanismos biológicos están involucrados en la disfunción muscular de los pacientes. Se proponen diversas pruebas para evaluar y diagnosticar el grado de afectación de los músculos respiratorios y de las extremidades (periféricos), así como identificar la capacidad de esfuerzo de los pacientes (prueba de marcha de 6min y cicloergometría). Se describen también las posibles estrategias terapéuticas vigentes que incluyen las diversas modalidades de entrenamiento y de soporte farmacológico y nutricional.
These guidelines discuss the latest findings on muscle dysfunction in patients with chronic obstructive pulmonary disease (COPD), and examine the general problem, etiology, diagnosis, evaluation, and treatment. To that end, the expert authors have summarized the most recent publications on the various aspects of the topic, and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) scale has been used to make recommendations on the grade of evidence for the various proposals discussed in this document.1 Due to space constraints, an extended version expanding on these guidelines has been made available online.
Epidemiology, Pathophysiology and Implications for PatientsMuscles in any part of the body have 2 main functional properties: strength, the maximum expression of their ability to contract; and endurance, the ability to maintain less than maximum strength over time.2 Strength depends mainly on muscle mass, while endurance is determined by the aerobic capacity of the muscle.3 Strength and endurance in different muscle groups can be measured in routine clinical practice.
Muscle dysfunction is defined as the inability of a muscle to perform its task,2 as a result of loss of strength or endurance, or both. Dysfunction of the respiratory muscles or the muscles of the limbs (also known as peripheral muscles) is common in respiratory diseases. Patients with limb muscle dysfunction lose their independence, which adversely affects their quality of life.4,5 COPD is probably the respiratory disease in which muscle dysfunction has been studied in most depth, and it has been established that up to one third of COPD patients, even in the early stages of the disease, have a loss of muscle function in their limbs (25% less strength than control subjects).4 Respiratory muscle dysfunction is seen in advanced COPD patients, whose diaphragmatic strength is between 20% and 30% of that of control subjects.6–8
Observational studies consistently show that COPD patients have muscle dysfunction, irrespective of the severity of their pulmonary obstruction. Evidence GRADE 1A.
Muscle Dysfunction PathophysiologyResearch over the last 20 years has revealed that several factors and mechanisms are involved in the multifactorial etiology of muscle dysfunction in COPD patients.
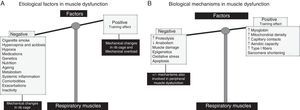
Limb Muscle Dysfunction (Quadriceps)As indicated in Fig. 1A, cigarette smoke, genetic and epigenetic alterations, metabolic disorders (including vitamin D and testosterone deficiencies), drugs (corticosteroids), comorbidities, exacerbations, systemic inflammation, malnutrition, physical inactivity, and aging are some of the factors involved in limb muscle dysfunction in COPD patients.2,3 The biological events implicated in limb muscle dysfunction most notably include a series of structural changes,9–11 oxidative stress,9,11,12 chronic hypoxia, hypercapnia and acidosis, and structural and mitochondrial changes13,14 (Fig. 1B). Other mechanisms, such as proteolysis, apoptosis, autophagy, and epigenetics, are also involved in the physiopathology of limb muscle dysfunction in these patients.9,15–19
(A) Etiological factors involved in limb muscle dysfunction in COPD patients. The factors have deleterious effects on lower limb muscle function and mass, causing the scales to completely tip toward the negative side (left tray). (B) In the lower limb muscles, the actions of the etiological factors are mediated by several biological mechanisms, with deleterious effects on muscle function, mass and structure. The scales are completely tipped to the left.
Observational studies consistently point to biological mechanisms and factors involved in the development of muscle dysfunction in COPD patients. Evidence GRADE 1A.
Respiratory Muscle DysfunctionThe major factors involved in the respiratory muscle dysfunction of COPD are shown in Fig. 2A. The most important of these are mechanical factors, but there are also factors that induce positive adaptation, which gives the respiratory muscles of these patients certain endurance3,20 (Fig. 2A). Adaptive biological phenomena have also been found in the diaphragm, counteracting the potential deleterious effects; these phenomena include shortening of the sarcomere length, increased myoglobin content and higher proportions of fatigue-resistant fibers and capillary contacts, increased mitochondrial density, and improved aerobic muscle potential3,21–25 (Fig. 2B). In COPD, the final muscle phenotype will be a result of the balance between the adaptive factors and mechanisms, and those involved in muscle function, as well as between stable disease and exacerbations (Fig. 2B). In advanced COPD however, biological mechanisms8,9,15–19,26 identical to those described in limb muscle dysfunction affect the diaphragm, prevailing over the adaptive mechanisms (Fig. 2B).
(A) In the respiratory muscles, etiologic factors such as alterations in the chest geometry and mechanical overload can to some extent counteract (training effect, right tray of the scales) the deleterious effects of the other etiologic factors of a more systematic nature (left tray of the scales), since these also contribute to limb muscle dysfunction. (B) In the respiratory muscles, several cell and molecular mechanisms have beneficial effects (adaptive mechanisms, right tray of the scales), which counteract the deleterious effects of the deleterious effects of the other biological mechanisms (left tray of the scales).
Observational studies consistently point to biological mechanisms and factors involved in the development of muscle dysfunction in COPD patients. Evidence GRADE 1A.
Evaluation of Respiratory Muscles: Voluntary and Involuntary Maneuvers (Table 1)Evaluating Respiratory Muscle Strength: Volitional Tests of Respiratory Muscle StrengthNon-Invasive TestingSpirometryForced spirometry,27 although non-specific, can detect a decline in forced vital capacity (FVC) that can be indicative of a non-obstructive ventilatory defect.28 A FVC decrease of greater than 25% between spirometries performed in the sitting and supine positions, or FVC less than 75% of the predicted values in the supine position, with normal values in the sitting position, indicate diaphragmatic weakness.29
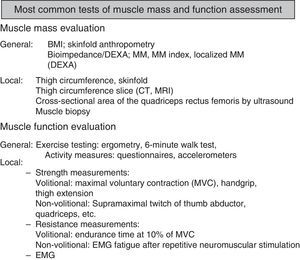
Types of Tests to Evaluate/Assess the Strength and Endurance Components of Muscle Function.
| Functional property | Muscle group | Test/Variable | Volitional | Pathological values | References |
|---|---|---|---|---|---|
| Strength | Non-specific | Forced spirometryPlethysmography | Yes | FEV1/FVC>80%TLC<80% pred. | 28,29 |
| Forced spirometry sitting to supine position | Yes | FVC sitting position and <75% pred. supineReduction >25% | 28,30 | ||
| Inspiratory | MIP | Yes | <80 pred. or <65% pred. or suspected if <−80cm H2O | 28,31 | |
| SNIP | Yes | Suspected if:Men: <−70cm H2OWomen: <−60cm H2O | 28,32,33 | ||
| Pesmax | Yes | Suspected if:Men: <−80cm H2OWomen: <−70cm H2O | 28,34 | ||
| Pdimax | Yes | Men: <−75cm H2OWomen: <−50cm H2O | 28,35 | ||
| Pdimax Twitch | No | Men: <−60 to 50cm H2OWomen: <−40 to 35cm H2O | 28,35 | ||
| Expiratory | MEP | Yes | <80% pred. or <65% pred. or suspected if <80cm H2O | 28,31 | |
| Upper limbs | Handgrip | Yes | <80% pred. | 55,56,57 | |
| Lower limbs (quadriceps) | Q-MVC | Yes | <80% pred. | 58,59 | |
| QTw | No | Not defined | 62,63 | ||
| Endurance | Non-specific | MSV | Yes | <60%–80% of MVV | 28,36 |
| Inspiratory | SMIP | Yes | Not defined | 37,38 | |
| Inspiratory tlim | Yes | Not defined | 37,38 | ||
| Expiratory | SMEP | Yes | Not defined | 39,40 | |
| Inspiratory tlim | Yes | Not defined | 39,40 | ||
| Quadriceps | Tlim at % QMCV | Yes | Not defined | 60 | |
| Pair of forces (torque) | Yes | Not defined | 61 |
Abbreviations. MIP, maximum inspiratory pressure at the mouth (static or Müller maneuver); MEP, maximum expiratory pressure at the mouth (static or Valsalva maneuver); SNIP, sniff nasal inspiratory pressure (dynamic sniff maneuver); Pesmax, maximum esophageal inspiratory pressure (generally obtained during sniff maneuver); Pdimax, diaphragmatic pressure on inspiration (generally obtained during sniff maneuver); Pdimax Twitch, transdiaphragmatic twitch pressure on inspiration, induced by electrical or magnetic stimulation; Q-MVC, maximal voluntary isometric contraction of the quadriceps; Qtw, contraction induced by twitch of the quadriceps muscle; MSV, maximum sustainable ventilation; MVV, maximal voluntary ventilation; SMIP, sustained maximal inspiratory pressure with incremental threshold loads; Tlim, time limit or endurance time for submaximal constant loads (generally percentage of SMIP or MIP for the inspiratory test or of SMEP or MEP for the expiratory test, and percentage of Q-MVC (generally 10%) for the quadriceps; SMEP, sustained maximal expiratory pressure with incremental threshold loads.
Maximum pressures generated at the mouth, whether inspiratory (MIP) or expiratory (MEP), are more specific for assessing respiratory muscle strength.27 These tests are generally static maneuvers (without airflow), performed using mouthpieces that can be occluded, with a small leak to prevent glottic closure and to reduce the use of the buccal muscles. MIP [usually determined from residual volume (RV)] is a measure of the strength of all the inspiratory muscles, while MEP [normally determined from total lung capacity (TLC)] basically expresses the strength of the expiratory muscles in the abdomen27 (Table 2). As for all voluntary maneuvers, the patient needs to be encouraged verbally, and three acceptable maneuvers should be obtained, with a variation of less than 20%. Reference values for the healthy Mediterranean population are available.30 MIP values greater than 80cm H2O can also exclude severe muscle dysfunction.27
Proposed Scale (John Moxham. Test of Respiratory Muscle Strength. UptoDate 2013) for Evaluating Patient Collaboration and Cooperation in Performing Maximum Pressure Maneuvers of Respiratory Muscles at the Mouth (MIP and MEP).
| Grades | |
|---|---|
| A | Excellent effort with excellent repeatability (<5cm H2O difference among maneuvers) |
| B | Good effort with good repeatability (5–10 cm H2O difference among maneuvers) |
| C | Fair effort with fair repeatability (10–20 cm H2O difference among maneuvers) |
| D | Only one good maneuver |
| F | Poor maneuvers or poor repeatability |
Nasal pressure is determined during maximal nasal inhalation (SNIP).27,31,32 This is a natural, dynamic maneuver, carried out with the patient in a sitting position, with one nostril blocked. It is usually measured from functional residual capacity (FRC),27,31,32 using the highest value from at least 10 maneuvers. Inspiratory muscle dysfunction can be ruled out if values are higher than −70cm H2O in men or −60cm H2O in women.27
Invasive TestingA good expression of the pleural pressure generated by activation of the inspiratory muscles can be obtained from the esophageal pressure determined by placing a pressure catheter in the mid-third of the esophagus during forced inhalation (sniffPesmax).27,33 Inspiratory muscle dysfunction can be ruled out if SniffPesmax values are higher than −80cm H2O in men or −70cm H2O in women.27
Diaphragmatic strength or transdiaphragmatic pressure (Pdi) can also be determined by measuring the difference between gastric and esophageal pressure, estimated by placing 2 catheters connected to pressure transducers (one in the esophagus and the other in the gastric cavity).27SniffPdi values lower than 75cm H2O in men or 53cm H2O in women suggest diaphragmatic dysfunction.34
The various studies consistently show that volitional testing for evaluating respiratory muscle function is useful and provides reliable inter-individual measurements over time. Evidence: GRADE 1A.
Non-Volitional Tests of Respiratory Muscle Strength (Table 1)External stimulation techniques, using phrenic nerve stimulators generating electric or magnetic fields (Pditwitch), have been developed to avoid false positives. Normal Pditwitch values are about 20%–30% of the sniffPdi.27,34
The different studies consistently show that non-volitional testing for evaluating respiratory muscle function is useful and provides reliable inter-individual measurements over time. Evidence: GRADE 1A.
Evaluating Respiratory Muscle Endurance (Table 1)Pressure–time product (PTP) is calculated by integrating the respiratory pressure measured at the mouth, esophagus or Pdi over time (cm H2O/min).27 Maximal sustainable ventilation (MSV) is expressed as a fraction of maximal voluntary ventilation (MVV); normal values are between 60% and 80% of the MVV.27 MSV is a measure of respiratory muscle endurance and the function of other elements of the rib cage.35 Inspiratory or expiratory threshold loading tests are more specific. There are two types: incremental, measuring the sustained maximal pressure (SMIP and SMEP, depending on whether inspiratory or expiratory), and constant submaximal loading (percentage of SMIP or MIP for inspiratory, and SMEP or MEP for expiratory).36–39
The different studies consistently show that endurance testing for evaluating respiratory muscle function is useful and provides reliable inter-individual measurements over time. Evidence: GRADE 1A.
Evaluation of Limb Muscles: Volitional and Non-Volitional TestingGeneral Evaluation of Limb Muscles (Fig. 3)Evaluating Muscle Mass (MM)Initial calculations are based on systems determining body compartments, such as the Matiegka method,40 which takes body weight (W) as the sum of 4 components: O, skeletal weight; D, skin and subcutaneous adipose tissue weight; M, skeletal muscle weight; and R, remaining weight. Skinfold anthropometry requires several measurements, including height, length and width of the limb, folds and calculated body surface area, but this technique generally overestimates the fat-free mass, compared to other methods.41 Technological advances have led to complex and even invasive systems, such as deuterium dilution,40 considered the gold standard. Although its use is limited to research centers, it has been used to validate a series of more commonly used measurements.
Bioimpedance Analysis (BIA) or Bioelectric ImpedanceThis method is based on the principle that the electrical conductivity of a fat-free mass (comparable to the MM) is greater than that of fat.42
Dual-Energy X-Ray Absorptiometry (DEXA)DEXA is an imaging technique used for estimating total-body and regional skeletal muscle mass. It also can be used for quantifying lean soft tissue, fat and bone mineral density.43
The different studies consistently show that the different tests for quantifying muscle mass in COPD patients are useful and provide reliable inter-individual measurements over time. Evidence: GRADE 1A.
Local MM Study TechniquesAlthough measuring thigh circumference is simple and cheap, it is not a good measure of local muscle mass.44 Alternatives include computed tomography (CT),44 magnetic resonance imaging (MRI),45 and ultrasound,46 particularly for measuring quadriceps size. In COPD, the amount of local MM measured by these techniques has been correlated with parameters as diverse as muscle strength47 and mortality.44 Muscle biopsy is a morphological technique that provides data on the structural and biochemical properties of the muscles,48 but its invasive nature means that use is essentially limited to research studies, even when fine needle microbiopsies are obtained.47
Numerous studies consistently show that obtaining a muscle biopsy from COPD patients is highly useful and provides reliable and comparable inter-individual measurements over time. Evidence: GRADE 1A.
Physical ActivityPhysical activity can be measured by questionnaires or, more reliably, by accelerometers that record activities performed by a subject over a prolonged period.49
Exercise TestingBoth the walk test and ergometry are discussed in detail in the specific section of these guidelines.
Electromyography (EMG)EMG consists of recording the electrical activity of muscles at rest and during both voluntary and externally stimulated contraction50–53 (see below).
The various studies consistently show that physical activity, exercise capacity and electromyography are useful and provide reliable and comparable inter-individual measurements over time. Evidence: GRADE 1A.
Limb Muscle Function TestingVoluntary ManeuversHandgrip DynamometryThis is a simple measurement, carried out with various types of dynamometers. It is widely used and is recognized as a prognostic factor in COPD.54 Reference values are available for different populations.55,56
Maximal Isometric Contraction of the Quadriceps Femoris (Q-MVC)This procedure is normally performed with the patient in a sitting position, with hips and knees bent at an angle of 90°. Quadriceps extension is measured with the leg fixed at the ankle, connected to a dynamometer.57 Reference values are available.58
Test of Endurance Time at 10% of the Q-MVCThis endurance test59 is based on claudication after exercise involving cycles of contractions against a load equivalent to 10% of the Q-MVC (cycles of 2s quadriceps contraction and 3s of relaxation). Other maneuvers, more specific for estimating endurance, are used for measuring pairs of forces (torque) in Nm (Newtons×meter).60
The different studies consistently show that tests for evaluating contraction function and muscle mass in COPD patients are very useful and provide reliable and comparable inter-individual measurements over time. Evidence: GRADE 1A.
Involuntary ManeuversSupramaximal Quadriceps Twitch Force (QTw)This technique is based on the electrical or magnetic stimulation of the femoral nerve in a single stimulus, or “twitch”, measuring the force generated after quadriceps activation.61,62
The various studies consistently show that involuntary maneuver tests for the evaluation of muscle function in COPD patients are useful and provide reliable and comparable inter-individual measurements over time. However, their use has not been extended to routine clinical practice. Evidence: GRADE 1B.
Evaluation of Exercise Tolerance: The 6-Minute Walk TestThe 6-minute walk test is a simple exercise protocol measuring the distance walked quickly by the patient on a hard, flat surface over a period of 6min.63 Several other tests are used in the field (stair-climbing, Bruce, 12-minute walk, incremental walk, etc.),63–65 but the 6-minute walk is the most popular, and is the most simple exercise test used as a reference in COPD and other diseases.66–72 It is highly standardized,63,73,74 is reasonably reproducible,75 and is valuable for measuring the effects of different interventions in many chronic diseases.76–81
The 6-minute walk is a high-intensity sustainable submaximal exercise82 used for the overall and comprehensive evaluation of various physical functions (cardiac, respiratory, peripheral oxygen transport, muscle bioenergetics and neuromuscular integration) that determine the patient's aerobic capacity. Some studies82,83 suggest that exercise intensity during the test is an indication of the patient's maximal sustainable power output over time (critical power).84 This would explain the high predictive value of this test for mortality76,79,80 and its valuable role in the clinical decision-making process.75,78,81
IndicationsEvaluation of the functional status of patients with different chronic diseases, such as COPD, asthma, obesity, interstitial lung diseases, pulmonary hypertension and various cardiovascular diseases66–72 and measurement of the effect of interventions, such as training, lung transplantation, lung parenchyma resection, and pharmacological treatments for pulmonary hypertension, COPD and heart failure were done.77,78 Although this is a very safe exercise protocol, since the patients themselves determine the speed of the walk, it should not be performed in patients with unstable angina, recent myocardial infarction (within 1 month), tachycardia of over 120bpm, or high blood pressure spikes. Safety measures and training by professionals are essential for any form of exercise testing.63
VariablesThe variables measured in the test are the following: (i) distance walked expressed in meters; (ii) heart rate and oxygen saturation measured by pulse oximetry at the beginning and end of the test; and (iii) symptoms, dyspnea (Borg scale) and discomfort in the lower limbs at the beginning and end of the walk. The walk test should be performed twice, with approximately 1h between assessments; the longest distance walked should be used. If the patient requires oxygen therapy, the test should be performed with the prescribed oxygen regimen in place.
Interpretation of ResultsFor comparison of the results obtained before and after an intervention, the minimum clinically important difference, set at 25–30m, must be identified after an intervention.66,69,70,85
Although results in terms of distance walked are usually interpreted as absolute values (meters), reference values are also used, such as those of Enright et al.86 In short, the 6-minute walk test is highly recommendable in clinical practice as a simple exercise test for evaluating functional status and the effect of certain interventions in patients with chronic respiratory and cardiac diseases. The simplicity, safety, reproducibility and high predictive value of the test make it a simple reference protocol for studying aerobic capacity in clinical practice.
The various studies consistently show that the 6-minute walk test is very useful and provides highly reliable and comparable inter-individual measurements over time. It is of great benefit in routine clinical practice and in clinical studies. Evidence: GRADE 1A.
Evaluation of Exercise Tolerance: CycloergometryIncremental Exercise TestingIncremental exercise testing can be used for evaluating the sub-maximal and maximal response, and can often help identify the mechanisms underlying exercise intolerance.87–90 It can also be used to adapt interventions for exercise intolerance to the source of the limitation,89–98 and for ruling out other medical interventions (such as physical training) that may be a risk factor, thus increasing patient safety.87,99
The procedure is highly standardized.64,87 Cycle ergometry is the most commonly used test, because it is cheap and compact, and the work rate can be accurately determined. Most patients can perform the test without practicing first.64,87,100
VariablesMost currently available software programs can generate large numbers of variables, but the most commonly used in clinical studies are maximum oxygen consumption (V˙O2max) and maximum work rate (WRmax), both of which are symptom-limited. V˙O2max is a highly reproducible variable, with coefficients of variation ranging from 3% to 7%, when maximum work rate criteria are reached.101–103 Consistent measurements have been obtained in multicenter clinical studies when appropriate quality controls are in place.104,105 Moreover, in cardiac patients at least, improved V˙O2max after interventions leads to improved survival.106V˙O2max is sensitive to both pharmacological and non-pharmacological interventions, and in particular to muscle training.11,107–111
The advantage of maximum work rate (WRmax) over V˙O2max is that it can be determined without the need for a metabolic cart. It should be remembered that, unlike V˙O2max, WRmax depends on the rate of the previous incremental protocol (ΔWR/Δt), so it cannot be equated with the V˙O2max. Moreover, exactly the same protocol must be used before and after the intervention or exposure.112
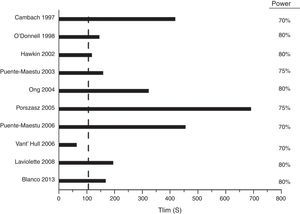
High-Intensity Constant-Power TestsAlthough moderate-intensity constant-power tests can be performed to characterize the kinetics of the response of the oxygen transport system, these tests require complex analysis, so their use is limited to research studies. In contrast, high-intensity constant-power (HICP) tests have been used widely in studies for assessing interventions targeting the muscles. The variable that defines exercise tolerance is time until the patient can no longer continue (Tlim, expressed in seconds). Tlim has been shown in multicenter trials to be reproducible105,113 and consistent105 (Fig. 4). The clinically important difference for these tests is in the order of 100s (95% confidence interval: 50–150s).113
The different studies consistently show that cycle ergometry is very useful and provides highly reliable and comparable inter-individual measurements over time. It is of great benefit in routine clinical practice and in clinical studies. Evidence: GRADE 1A.
Muscle Training in COPD Muscle DysfunctionMuscle training, a fundamental component of Pulmonary Rehabilitation (PR) programs, has been widely shown to be effective for improving exercise tolerance, muscle strength, dyspnea, fatigue, and quality of life. Hence, it is an essential intervention in the treatment of muscle dysfunction in COPD patients.2,114
In patients with chronic respiratory disease, general muscle training must be aimed at improving both the aerobic limitations and muscle dysfunction often seen in these diseases. Choice of training type will not only depend on the needs of the patient and planned objectives, but also to a large extent on the resources available in the rehabilitation clinic.115,116
The various studies consistently show that muscle training is the best treatment for muscle dysfunction in COPD patients. It is of benefit in routine clinical practice and in clinical studies. Evidence: GRADE 1A.
Types of TrainingAerobic or Endurance TrainingThis is the type of training most often used in PR, and the evidence for recommendation is the highest (Grade 1A).2,114,117,118 Aerobic exercise is a submaximal exercise involving the large muscle groups, sustained for a prolonged length of time. In COPD patients, aerobic training not only achieves better cardiovascular adaptation, it also improves limb muscle function. This leads to increased muscle endurance, with adaptive phenomena in the bioenergetics of the quadriceps119,120 and skeletal muscles.121
Cycloergometry and treadmill training are the most common aerobic exercises prescribed in PR, especially in outpatient and inpatient rehabilitation programs. Training intensity is very important when prescribing exercise therapy. An intensity of around 60%–80% of the maximal exercise capacity (previously evaluated with cardiopulmonary exercise testing) for a minimum of 8 weeks118 is recommended for achieving a substantial benefit, while 12 weeks is considered the optimal duration114 (Table 3). Longer exercise programs can achieve greater, longer-lasting effects, particularly on quality of life indices.122
Practical Recommendations for Aerobic and Strength Training in COPD.
| Aerobic/Endurance training | Strength training | |
|---|---|---|
| Objective | Improve aerobic capacity. Improve limb muscle function | Overload large upper and lower limb muscle groups. Increase muscle strength and endurance |
| Frequency | 3–4 days/week | 2–3 days/week |
| Mode | 20–30min | 2–4 series of 6–12 repetitions |
| Intensity | 60%–80% of WRmax | 70%–85% of 1 RM |
| Duration | 8–12 weeks | 8–12 weeks |
The various studies consistently show that aerobic endurance muscle training is one of the best treatments for muscle dysfunction and restoring exercise capacity in COPD patients. It is of benefit in routine clinical practice and in clinical studies. Evidence: GRADE 1A.
Interval TrainingThis is a modification of standard aerobic training, in which short periods (1–2min duration) of high-intensity exercise (80%–120% maximum capacity) are regularly alternated with similar periods of rest or lower intensity exercise. In this way, patients achieve high levels of output, but with less dyspnea and fatigue. The benefits are equivalent to those of conventional aerobic training.121,123,124
The different studies consistently show that interval training is one of the best treatments for muscle dysfunction and restoring exercise capacity in more severe COPD patients. It is of benefit in routine clinical practice and in clinical studies. Evidence: GRADE 1A.
Strength TrainingFollowing the “specificity principle”, muscle strength training is potentially better for increasing the strength and mass of the exercised muscle than conventional aerobic training.125 Available evidence supports the use of strength training in combination with general aerobic training (1A), since this achieves further increases in limb muscle strength.114,118 In PR, this type of exercise is often applied with weight-lifting exercises for the lower and upper limbs, performed on gym apparatus with heavy loads, equivalent to 70%–85% of the maximum weight lifted by the individual in a previous single maneuver (or one repetition maximum testing, 1 RM), with few repetitions,118 in 2–3 sessions per week for 8–12 weeks.114,118
The different studies consistently show that muscle strength training is one of the best treatments for muscle dysfunction and restoring exercise capacity in more severe COPD patients. It is of great benefit in routine clinical practice and in clinical studies. Evidence: GRADE 1A.
Other Types of TrainingVery promising alternatives for limb muscle training in PR programs include transcutaneous electrical nerve stimulation (TENS)2,126,127 and electromagnetic stimulation,128 which is better tolerated but less well studied.
Respiratory Muscle TrainingInspiratory muscle training (IMT) has been shown to improve muscle strength and endurance in COPD patients, with benefits in dyspnea, functional capacity and quality of life.129 The efficacy of specific expiratory muscle training with abdominal muscle exercises remains in doubt. In general, IMT should be performed twice a day, at an intensity of at least 30% of the MIP and in sessions lasting about 15min.118 IMT is indicated mainly in patients with respiratory muscle dysfunction.
The various studies consistently show that respiratory muscle training may be of benefit in COPD patients with significant respiratory muscle dysfunction, in routine clinical practice and in clinical studies. Evidence: GRADE 1B.
Other Non-Training-Based Treatments for Muscle DysfunctionNutritional Supplements and Hormone TreatmentNutritional disorders are found in 20%–40% of patients with severe respiratory disease. The causes appear to be multifactorial, and include imbalance between energy intake and output, hypoxemia, and the systemic inflammatory component often observed with this disease.130–132 Results from the use of high-energy nutritional supplements in these patients (anabolic steroids and growth hormones, antioxidants and other protein compounds) have been contradictory as regards weight gain and, in particular, their impact on functional recovery.
Nutritional SupplementsNutritional supplements are commonly used in COPD patients, but there is very little evidence to support this approach. While some authors have reported weight gain and increased fat-free mass, along with improvements in exercise tolerance, these data are not supported by other studies. The most recent Cochrane review133 includes a meta-analysis of 17 randomized clinical trials,134–148 performed mainly in undernourished patients, that provide little evidence that patients receiving nutritional supplements show improvements in weight, fat-free mass, respiratory muscle strength and distance walked during the 6-minute walk test.
Studies do not consistently show that the benefits outweigh the risks of treatment administration. It is hoped that better quality research in the future will contribute to improved understanding in this area, and provide more reliable results than those currently available, to help introduce this type of treatment into routine clinical practice for the management of COPD patients. Evidence GRADE 2B.
Anabolic SteroidsAndrogen deficiency, often associated with loss of muscle mass, occurs in up to 40% of COPD patients.149 Administration of anabolic steroids has been associated with weight gain and increased muscle mass, supporting the use of these products as adjuvant treatment in rehabilitation programs. However, the results have not been sufficiently corroborated, and this approach is not recommended by ERS/ATS rehabilitation guidelines.114 Several published studies have shown that despite weight gain and increased fat-free mass, no improvements were reported in lung function parameters, exercise tolerance or respiratory (MIP) or limb (handgrip strength) muscle function.143,150–155 However, the meta-analysis included not only treatment with androgenic derivatives, but also a study on growth hormone and another on ghrelin (a growth hormone secretagogue), as well as other studies that may or may not have included training, with no subgroup analyses. All these factors limit the interpretation of the above-mentioned meta-analysis. In reality, the combination of nutritional or androgenic supplements with muscle training has led to increases in weight, fat-free muscle mass, MIP, quadriceps force, maximum work load and exercise endurance time.156
The studies do not consistently show that the benefits outweigh the risks of treatment administration. It is hoped that better quality research in the future will contribute to improved understanding in this area, and provide more reliable results than those currently available, to help introduce this type of treatment into routine clinical practice for the management of COPD patients. Evidence GRADE 2B.
Growth Hormone and GhrelinThe use of recombinant human growth hormone (GH) has also been studied in malnourished COPD patients, in rehabilitation programs, but results have been inconclusive.150,157 More recently, the use of ghrelin has been studied in these patients, also in the rehabilitation setting. Ghrelin is polypeptide hormone that acts by binding to GH-stimulating hypothalamic receptors, stimulating secretion of GH. It also stimulates the release of orexigenic factors, increasing appetite.158 Paradoxically, malnourished COPD patients may have elevated ghrelin levels.159 Results published to date on the exogenous administration of this substance are inconclusive.160–162
The studies do not consistently show that the benefits outweigh the risks of treatment administration. It is hoped that better quality research in the future will contribute to improved understanding in this area, and provide more reliable results than those currently available, to help introduce this type of treatment into routine clinical practice for the management of COPD patients. Evidence GRADE 2C.
Other NutrientsThe ability of other products to improve the muscle and physical function of COPD patients, including antioxidants, vitamins159 and high-calorie compounds, has been investigated. However studies are generally isolated and uncontrolled, or conducted in small numbers of patients, and results are difficult to reproduce.163,164
The studies do not consistently show that the benefits outweigh the risks of treatment administration. It is hoped that better quality research in the future will contribute to improved understanding in this area, and provide more reliable results than those currently available, to help introduce this type of treatment into routine clinical practice for the management of COPD patients. Evidence GRADE 2C.
Non-Pharmacological Treatments: Non-Invasive Ventilation and HelioxIt is well known that non-invasive mechanical ventilation (NIV) allows the respiratory muscles to rest, explaining improved lung function, evidenced by increased MIP, Pdimax and pressure-time product. A few studies have even associated the use of NIV with better limb muscle function, demonstrated by increased quadriceps strength and endurance.165 This is probably a consequence of improved oxygen supply to the peripheral muscles, as a result of the reduction in work-of-breathing.166 This same mechanism has been cited to explain the effect of breathing heliox (a mixture of helium and oxygen) on limb muscle function. A recent Cochrane review, based on a meta-analysis of 6 clinical trials evaluating the results of NIV during a physical exercise program, concluded that the slight differences in exercise capacity and tolerance were inconsistent. In line with the latest consensus recommendations from the ERS/ATS, the use of NIV during muscle training is not recommended.114 This intervention should be limited to hypercapnic patients on night-time NIV programs.
The studies consistently show that the benefits outweigh the risks of treatment administration. Research in the future may contribute to improved understanding in this area, to help introduce this type of treatment into routine clinical practice for the management of COPD patients. Evidence GRADE 2A
ConclusionsIn summary, good respiratory and limb muscle function is essential for sustaining life and an acceptable quality of life. Muscle function is affected in many respiratory diseases and may lead to problems for the patient's social life and for maintaining an adequate level of ventilation. Muscle function can and must be evaluated in these patients, so that the appropriate therapeutic measures can be implemented. In view of the current evidence, muscle training, with or without nutritional supplements, is clearly the best therapeutic strategy for increasing muscle mass and function and improving quality of life in COPD patients.
The authors thank Ester Puig-Vilanova for her support and Anael Barberán-García for her invaluable contribution to the review of the literature and incorporation of references. They also thank Anna Salazar, María Cortés-Badia and Paula Bassagañas-Òdena for their collaboration in producing the list of abbreviations and references in the document.
Please cite this article as: Barreiro E, Bustamante V, Cejudo P, Gáldiz JB, Gea J, de Lucas P, et al. Normativa SEPAR sobre disfunción muscular de los pacientes con enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2015;51:384–395.