The evaluation of airway obstruction is fundamental in the management of asthma and COPD. Obstruction is usually quantified by spirometry, comparing FEV1 and FEV1/FVC with reference values.1 A reduced expiratory flow caused by obstruction of the small airways produces a concave pattern on the expiratory flow–volume curve (EFVC) in forced exhalation.2 The standard practice of direct observation is subject to a certain degree of variability. As an alternative, if high quality maneuvers are obtained,1 the graphic properties of this curve can be measured objectively, although this method is relatively unknown in the literature and standard practice.3 The additional information provided by this method should increase the sensitivity of the diagnosis of obstruction, especially in patients with normal values on standard spirometry.
In this study, we describe the different techniques for measuring bronchial obstruction using curvilinear graphical analysis.
We performed a non-systematic review of the literature in the Pubmed database using the terms “concavity”, “curvilinearity” and “spirometry”. Prospective and retrospective search techniques were then applied. Studies were selected that assessed the diagnostic and prognostic capacity of curvilinear analytical methods.
We retrieved 13 articles describing 4 methods of evaluating obstruction using EFVC analysis.
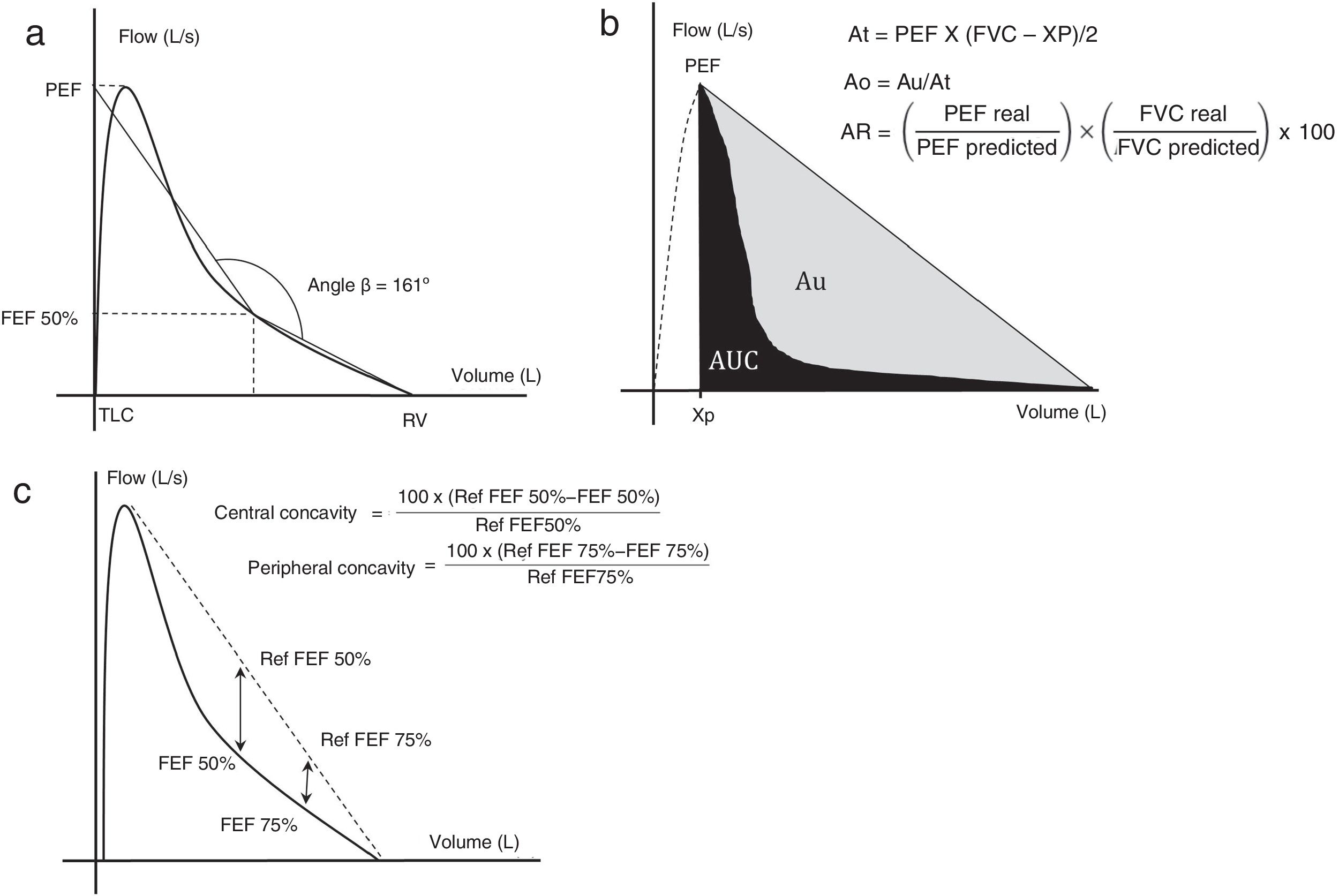
The angle beta (Aβ) was created3 in order to quantify the level of concavity of the EFVC. To this end, two straight lines were plotted: one from the point of residual volume to the EFVC at 50% of expiratory volume and one from 50% expiratory volume to the extrapolation of the peak expiratory flow on the vertical axis, corresponding to total lung capacity (Fig. 1a). Aβ is measured at the point where these two straight lines intersect.
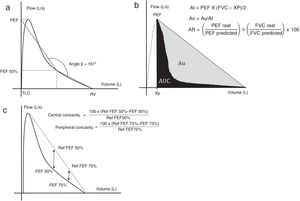
(a) Measuring angle β. (b) Area under the curve method. (c) Central and peripheral concavity. Each method is described in the text.
Ao: obstructive area; AR area: area of the rectangle; At: area of the triangle; Au: area between the hypotenuse of the triangle and the expiratory forced expiration curve; AUC: area under the curve; FEF50%: forced expiratory flow at 50% of vital capacity; FEF75%: forced expiratory flow at 75% of vital capacity; L/s: liters per second; PEF: peak expiratory flow; Ref: reference; RV: residual volume; TLC: total lung capacity; Xp: extrapolation of PEF in the abscissa.
Graphics adapted from Dominelli et al.,5 Lee et al.,11 and Johns et al.15
In adult patients, Aβ has been used to quantify obstruction in patients with asthma4 and COPD.3,5 Reference values for pediatric patients have been described.6 One study showed a 91% specificity for distinguishing patients with atopic asthma from healthy controls.7 A lower Aβ measured by z-score was observed in patients with wheezing and spirometric obstruction than in healthy patients.8
An improvement in the Aβ in adults with asthma was recorded after treatment with inhaled corticosteroids9 and bronchodilators.4 A study in pediatric and adult patients10 showed a significant correlation (r=−0.959) with the visual estimation of concavity by experts.
The degree of obstruction can be estimated by the area under the curve (AUC).11,12 To achieve this, the area of a right-angled triangle (At) with one cathetus originating from the extrapolation of the peak expiratory flow (PEF) at the level of the abscissa (Xp) and the other running from this point to the point of residual volume, with the hypotenuse connecting both ends is calculated (Fig. 1b). Specific software is used to determine expiratory AUC, which, when subtracted from the At can be used to quantify the area Au (between the hypotenuse of the triangle and the EFVC). Given that this area depends on the degree of concavity and the anthropometric characteristics of the subject, the obstructive area (Ao=Au/At), which is proportional to the degree of obstruction, is used.
Using the same catheti mentioned above, the area of the rectangle (AR) can be calculated from the ratio between a real and a predicted rectangle.
By studying these variables and their ratios, both Ao/AR and Ao/PEF were found to correlate closely with RV/TLC (r=0.718 and r=0.780, respectively; P=.001 for both) and distance walked on a 6-minute walk test (r=−0.618 and r=−0.581, respectively; P<.01 both).11 Furthermore, in a study of AUC/At in the first 3s of expiration,12 a strong correlation (r=0.88; P<.001) was observed with obstruction measured by FEV1/FVC and a kappa of 0.72 for the diagnosis of COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.
EFVC concavity can be quantified using mathematical models. Hyperbolic13 and quadratic14 functions have been used to estimate the curve. The greatest difference between functions is that in one, maximum curvature (Kmax) is estimated while in the other, the average curvature index (ACI) is determined.
A negative exponential relationship between Kmax and FEV1 has been found in a heterogeneous population of adults.13 In pediatric patients with asthma, moreover, a regular correlation (r=0.53; P<.001) was observed between ACI and symptoms that was higher than that of the traditional spirometric variables (r=−0.22 for FEV1; P=.14).14
Concavity indices are calculated by comparing forced expiratory flows (FEF50% and 75%) with the reference values (estimated by extrapolation of their points toward a straight line that joins PEF with the point of residual volume) (Fig. 1c).
A study of non-smokers15 showed a higher prevalence of obstruction according to central (12%–14.6%) and peripheral (14.6%–17.9%) concavity compared with FEV1 (6.2%–8.0%), and FEV1/FVC (5.6%–8.3%) that was not associated with clinical outcomes, suggesting possible overdiagnosis. There was a high correlation (between r=−0.710 and −0.789) for concavity indices and FEV1/FVC.
Curvilinear analysis techniques showed variable usefulness in the diagnosis of obstruction, association with therapeutic response, and the presence of symptoms. The populations evaluated were heterogeneous in terms of etiology and age range. No comparisons were made between methods, and the methods for demonstrating diagnostic utility vary among the studies. The methods involve a risk of overdiagnosing obstruction, and standardized limits are not available for some. The pediatric population may benefit from the use of these techniques, since their standards of obstruction are less sensitive.
The wider dissemination of these methods could lead to new applications in medical practice. However, there is not enough evidence to recommend any method in a systematic way. It would be useful to evaluate these methods in symptomatic patients without spirometric obstruction with the aim of detecting a population who might benefit from treatment.
Please cite this article as: Maritano Furcada J, Rodríguez CI, Wainstein EJ, Benito HJ. Métodos de análisis gráfico de obstrucción espirométrica: ¿una imagen vale más que mil palabras?. Arch Bronconeumol. 2019;55:272–274.