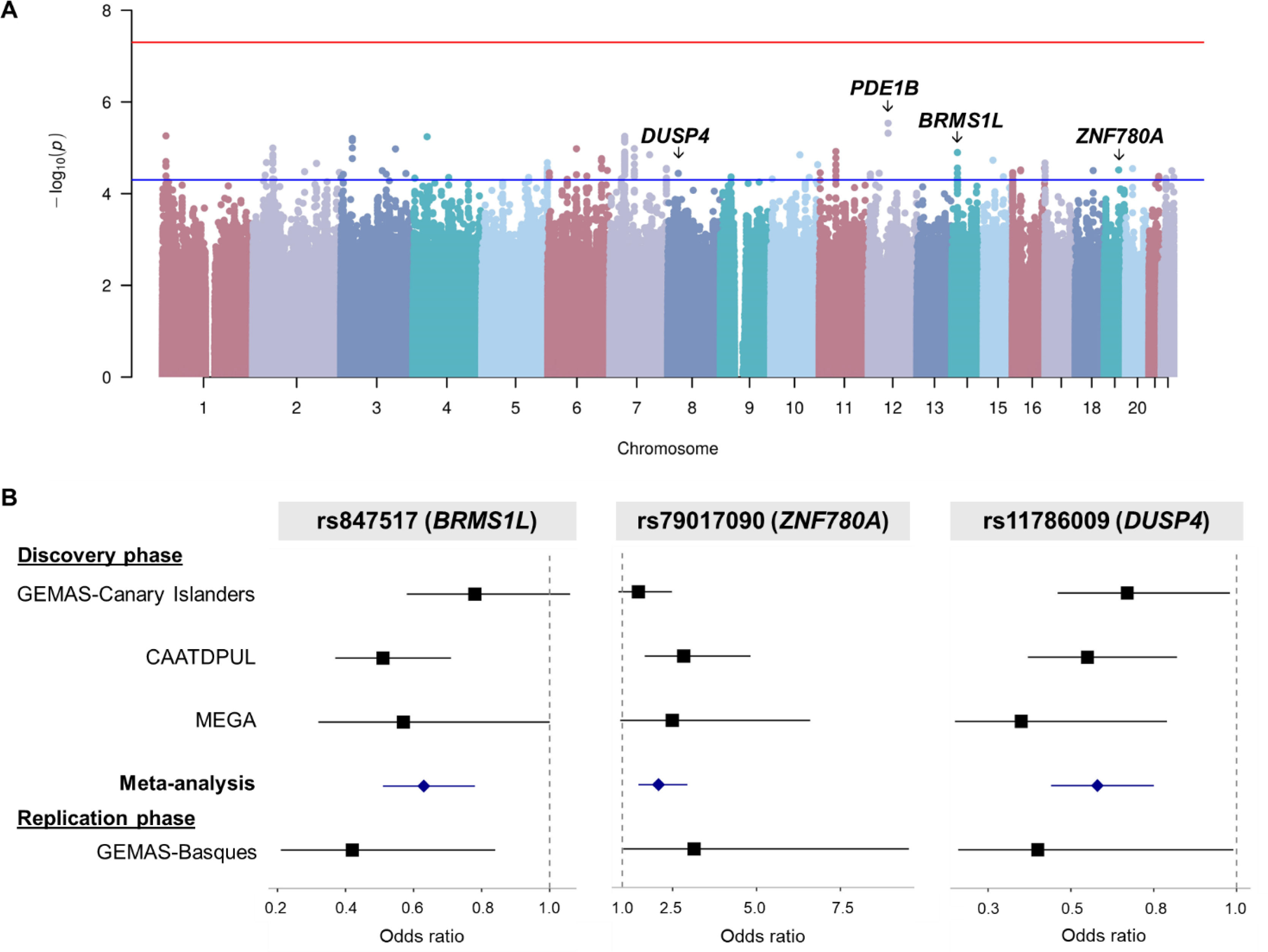
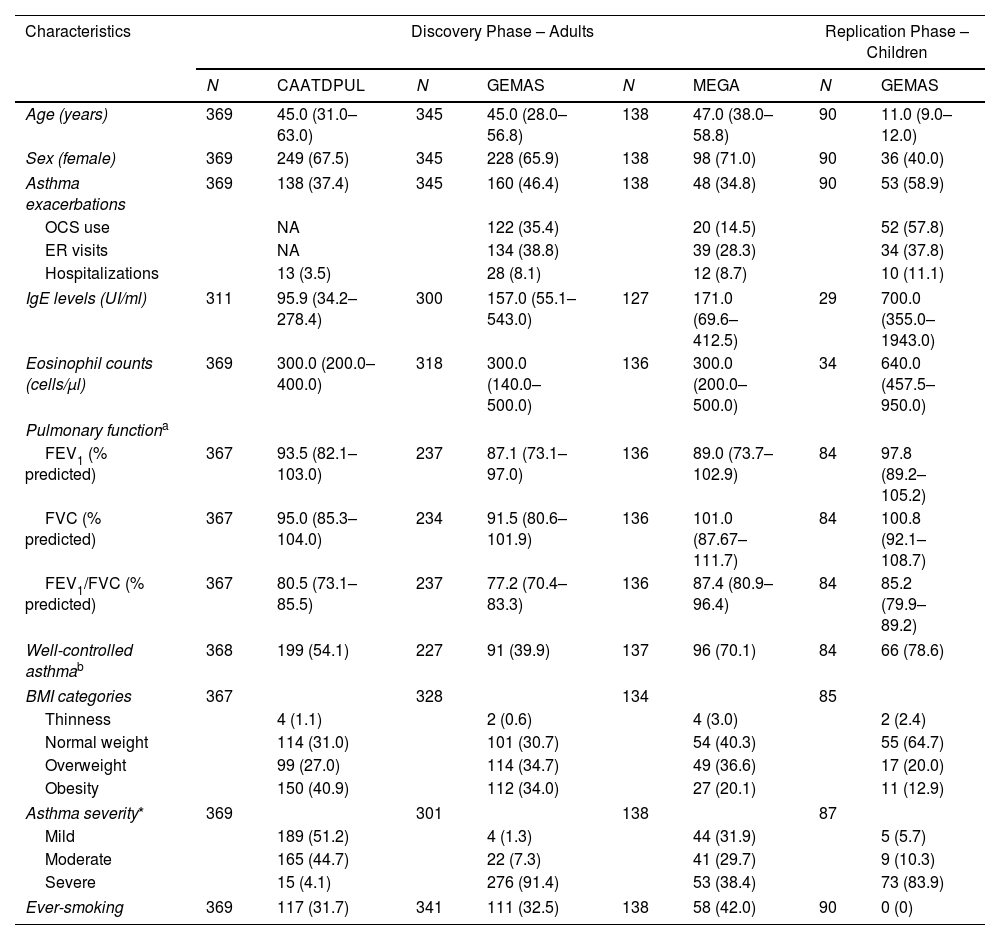
Archivos de Bronconeumologia is an international journal that publishes original studies whose content is based upon results of research initiatives dealing with several aspects of respiratory medicine including epidemiology, respiratory physiology, pathophysiology of respiratory diseases, clinical management, thoracic surgery, pediatric lung diseases, respiratory critical care, respiratory allergy and translational research. Other types of articles such as editorials, reviews, and different types of letters are also published in the journal. Additionally, the journal expresses the voice of the following scientific societies: the Spanish Respiratory Society of Pneumology and Thoracic Surgery (SEPAR; https://www.separ.es/), the Latin American Thoracic Society (ALAT; https://alatorax.org/), and the Iberian American Association of Thoracic Surgery (AIACT; http://www.aiatorax.com/).
It is a monthly journal in which all manuscripts are sent to peer-review and handled by the editor or an associate editor from the team and the final decision is made on the basis of the comments from the expert reviewers and the editors. The journal is published solely in English. All the published data is composed of novel manuscripts not previously published in any other journal and not being in consideration for publication in any other journal..
The journal is indexed at Science Citation Index Expanded, Medline/Pubmed, Embase and SCOPUS. Access to any published article is possible through the journal's web page as well as from Pubmed, ScienceDirect, and other international databases. Furthermore, the journal is also present in X, Facebook and Linkedin. Manuscripts can be submitted electronically using the following web site: https://www.editorialmanager.com/ARBR/.