The present study intends to describe the characteristics of patients diagnosed with severe alpha-1 antitrypsin deficiency (AATD) in Spain, to observe the rate of decline in forced expiratory volume in 1s (FEV1) with and without substitutive therapy, and to identify factors associated with a rapid rate of decline in FEV1.
MethodA retrospective study of the evolution of individuals with AATD was carried out based on data collected from the Spanish registry. The primary response variable was the annual rate of decline in FEV1, calculated using the baseline and last postbronchodilator FEV1 values in an endpoint analysis.
ResultsThree hundred and three patients with severe AATD and Pi ZZ phenotype were identified. Follow-up spirometric data were collected for 117 subjects. Being a smoker or ex-smoker vs never smoker (odds ratio [OR]=10.31; 95% confidence interval (CI)=1.8–58.8; P=.008) and having a higher baseline postbronchodilator FEV1 (% predicted) (OR=1.03; 95% CI=1.005–1.06; P=.018) were independently associated with a more rapid rate of decline in FEV1. There was also a trend towards a relationship between low body mass index (BMI) and a greater rate of deterioration in lung function (OR=1.14; 95% CI=0.98–1.33; P=.085).
ConclusionBeing a smoker or ex-smoker, greater baseline lung function, and low BMI were the main risk factors associated with an accelerated rate of decline in FEV1. This finding warrants the close observation of younger patients with a better-preserved FEV1.
El presente estudio pretende describir las características de los pacientes diagnosticados de déficit grave de alfa-1-antitripsina (AAT) en España, calcular la tasa de descenso del FEV1 con y sin tratamiento sustitutivo, e identificar factores asociados a una tasa de descenso acelerada del FEV1.
MétodoEstudio retrospectivo de la evolución de los individuos con déficit de alfa-1-antitripsina (DAAT) incluidos en el registro español. La variable principal evaluada en el estudio fue la tasa anual de descenso del FEV1.
ResultadosSe identificaron 303 pacientes con DAAT grave y fenotipo Pi ZZ. Se dispuso del seguimiento espirométrico de 117 pacientes. Ser fumador activo o ex fumador frente a nunca fumador (odds ratio [OR]=10,31; intervalo de confianza (IC) del 95%=1,8–58,8; P=0,008) y tener un mayor FEV1(%) posbroncodilatador (OR=1,03; IC del 95%=1,005–1,06; P=0,018), se asociaron de manera independiente a una tasa más acelerada de descenso del FEV1. Se apreció una tendencia entre tener un índice de masa corporal (IMC) bajo y experimentar una mayor tasa de deterioro del FEV1 (OR=1,14; IC del 95%=0,98–1,33; P=0,085).
ConclusionesSer fumador o ex fumador, tener una función pulmonar preservada y un bajo IMC fueron los principales factores de riesgo asociados a una tasa acelerada de descenso del FEV1. Este hallazgo justificaría la necesidad de efectuar un seguimiento estrecho de los pacientes jóvenes con un FEV1 más preservado.
Alpha-1 antitrypsin (AAT) is a highly pleomorphic glycoprotein belonging to the serpin superfamily, with more than 100 varieties and whose main characteristic is its antiprotease function, especially anti-neutrophil elastase.1,2
The normal allele, present in more than 90% of the population, is called PI*M. The most frequent deficient allelic variants are PI*S and PI*Z, which are responsible for the production of abnormal proteins that polymerize within the hepatocytes; thus, the plasma levels are markedly reduced in subjects who are carriers of at least one and especially two Z deficiency alleles.3,4 AAT deficiency (AATD) is one of the most common genetic disorders. It is the main genetic factor that contributes to the development of pulmonary emphysema in adults, as the absence of AAT causes an imbalance favoring proteases, which cause tissue damage.
AATD, defined by serum concentrations of AAT less than 35% normal values, is a rare condition that affects one out of every 2000–5000 Caucasian individuals, descendants of Northern, Central, and Western Europeans.5 As occurs in other rare diseases, patient registries have been developed in order to compile information and, in this manner, improve the understanding of the disease. Although clinical assays are considered the gold standard for establishing the most effective interventions, the information found in the registry and from other observational studies can be used to fill in important gaps that, if not, would be left uncovered because clinical assays do not deal with real-life situations.6
The world-wide AATD population registries (Alpha One International Registry [AIR], the registry of the National Heart, Lung, and Blood Institute [NHLBI] in the United States and the Alpha One Foundation Research Network Registry [AOF-RNR]) have demonstrated that the main clinical characteristic of this disease is effort dyspnea.7,8 They have also shown that the majority of patients are diagnosed when they have already developed severe lung disease, generally at an earlier age than patients with chronic obstructive pulmonary disease (COPD) without AATD.
The evolution in clinical terms and life expectancy are both directly related with the accelerated loss of forced expiratory volume in 1s (FEV1), and it has been reported that the lung function deterioration is faster in individuals with AATD than in COPD patients without AATD.9–13
Several studies have shown that smoking and respiratory exacerbations are the main factors related with the deterioration of FEV1. They have also suggested that substitutive treatment (intravenous administration of purified AAT from blood donors, capable of maintaining AAT plasma levels above 80mg/dl) could stop the decline of FEV1, reduce the frequency of the exacerbations and even stop the loss of lung density.14–17
The natural history of AATD is not well known. Its clinical and functional impacts are not homogeneous in all individuals and the existing series include very diverse populations that are occasionally very limited.18 Health-care institutions and scientific societies recognize that the creation of rare disease registries is an essential strategy in order to be able to develop clinical studies and clarify the natural history of these diseases. The Spanish registry of patients with alpha-1 antitrypsin deficiency, or REDAAT, compiles the clinical and functional data of the Spanish population with AATD. REDAAT is a Spanish national registry and was founded in 1993 as a workgroup of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).19
The present study describes the evolution of FEV1 in patients belonging to REDAAT, centered on the parameters that could potentially have a predictive value for a fast deterioration in lung function.20
MethodsBased on the information compiled in the REDAAT database, we carried out a retrospective study in patients with AATD. The objectives of this study were: (a) to describe the characteristics of the patients diagnosed with AATD in Spain; (b) to observe the rate of decline in FEV1 of the patients who had been or currently were receiving substitutive treatment and those who had/were not; and (c) to identify the factors associated with a greater rate of descent in FEV1.
ParticipantsThe data included in this study come from subjects with AATD included in REDAAT. REDAAT can be accessed through its web page (www.redaat.es), which meets all the requirements related with the protection of personal data in accordance with Spanish legislation. Its page is certified as an Accredited Medical Web by the commission of the College of Physicians of Barcelona. Although the recommendation of REDAAT is to include data on the follow-up visits biannually, information on the evolutional follow-up of the majority of the registered individuals is not available. In order to achieve the objectives of the study, we contacted all the doctors who collaborated with the registry by mail, e-mail and/or telephone requesting them to provide data related to the follow-up of their patients. The data obtained were included anonymously in a database in Access format, and the quality was checked, as was the frequency of absent data. The study was done in accordance with the principles of the Declaration of Helsinki, the guidelines for good practice in epidemiological research and the local regulations for the use of study databases.
The inclusion criteria for the study were: (a) severe AAT deficiency, demonstrated by serum levels of AAT lower than 50mg/dl with phenotype Pi ZZ or other deficient allelic variants, such as PiBarcelona21; and (b) to have at least 2 spirometries available over a time period of more than 6 months in order to analyze the rate of decline in FEV1. The exclusion criteria were the presence of other non-severe deficient phenotypes, heterozygous Pi MZ, Pi MS, and Pi SZ patients and those who had undergone transplant or lung volume reduction surgery.
MeasurementsThe REDAAT database has the same variables as the international registry (Alpha One International Registry [AIR])8 and includes sociodemographic parameters (such as age, sex, weight, tobacco habit, and accumulated tobacco consumption) as well as the reason for the determination of the AAT levels, due to either the existence of a related pathology (lung disease, liver disease, etc.), existence of a family history or population screening programs.
The spirometries were done at each participating center and were reviewed and remitted by specialists in Pneumology. The repeated measures for each patient were always done in the same center.
Information was also compiled on the following clinical variables: presence of emphysema, bronchiectasis, and asthma; age at onset of the symptoms; main semiology; and previous history of pneumonia. Among the most relevant comorbidities are diabetes mellitus, cardiovascular disease and renal or hepatic failure according to the codification of CIE-9. The database includes therapeutic data related with the lung disease (pharmacological treatment and chronic oxygen therapy) and procedures such as lung or liver transplantation and lung volume reduction surgery.
It was also documented whether the patient had received at some time or was receiving substitutive treatment. In Spain, the indications for substitutive treatment are the following: severe AAT deficiency (phenotype ZZ or another rare deficiency) with deteriorated lung function or evidence of an accelerated loss in lung function as measured by forced spirometry.22 Based on pharmacokinetic studies, the recommended dosages are: 60mg/kg/7 days, 120mg/kg/15 days, and 180mg/kg/21 days, with an adjustment in the dose depending on the valley levels of plasma AAT.23
Statistical AnalysisThe main variable analyzed was the annual rate of decline in FEV1 expressed in ml/year. For the analysis of this variable, the difference between final and baseline FEV1 (ΔFEV1 final-baseline) was calculated. The difference, obtained in ml, was divided by the number of follow-up months and was multiplied by 12 in order to obtain the fall in ml/year in an initial–final analysis, following the method used in the study by Dirksen et al.24 The baseline value was defined as the greater of the first 2 measurements, spaced by less than 6 months, as it was observed that some patients experienced a significant improvement in the lung function in the interval of time between 2 consecutive spirometries due to the optimization of the COPD treatment after its diagnosis.
The baseline characteristics were reported in frequency tables (categorical variables) and measurements of central tendency or dispersion (continuous variables). Due to the small sample size, non-parametric tests were used. The continuous variables were compared with the Mann–Whitney U-test or the Wilcoxon/Kruskal–Wallis test (analysis of variance method using non-parametric tests) depending on the groups to be compared. The categorical variables were compared using either the χ2 or Fisher's exact test.
Likewise, a multivariate analysis was performed in order to identify factors associated with the rapid decline in lung function by means of a stepwise logistic regression model. To do so, the main variable was created, classifying the sample as the subgroup of patients with accelerated loss (grouping the values lower than this value in the first tertile) or slow loss (values higher than the value of the third tertile). The following variables were included: age, sex, baseline post-bronchodilator FEV1 (in % of predicted), smoker (active or ex-smoker) or non-smoker, body mass index (BMI), history of asthma or pneumonia, number of years in follow-up, number of FEV1 measurements throughout the follow-up, and whether or not substitutive treatment was received. The statistical analysis was carried out with version 17 of the SPSS® program and the previously set level of statistical significance was 0.05.
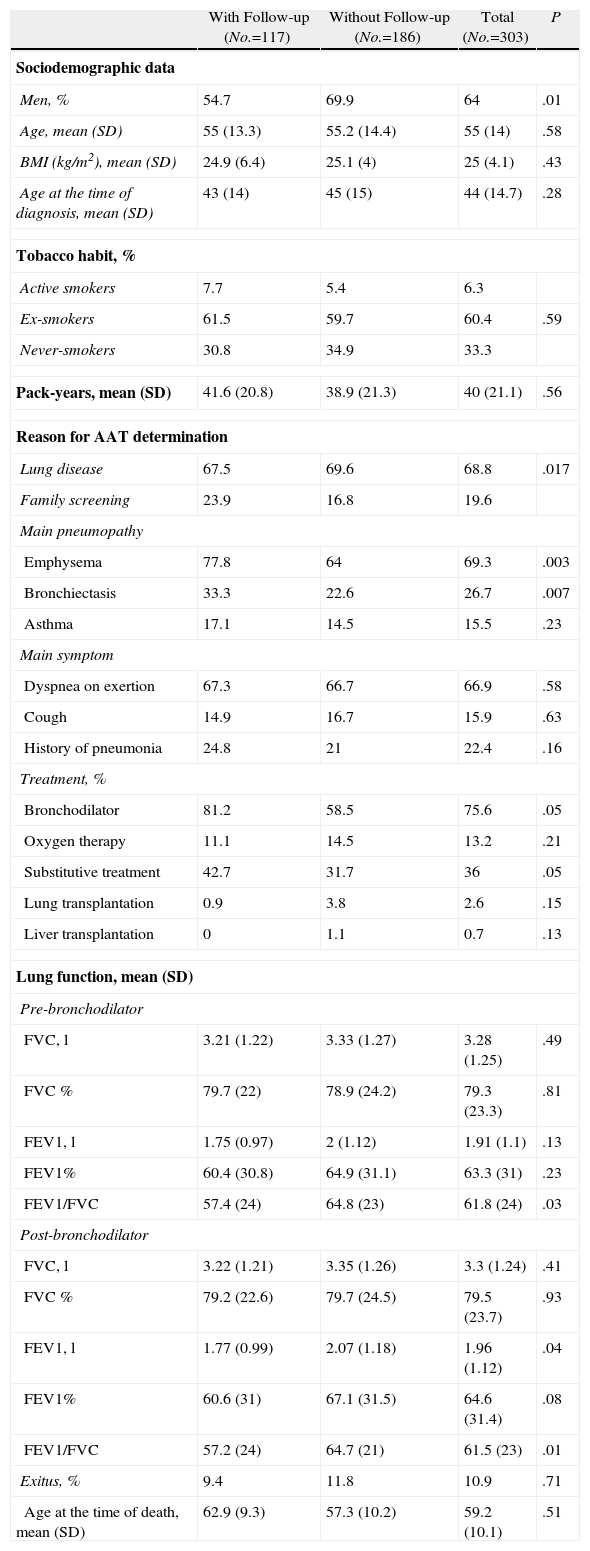
ResultsBaseline CharacteristicsOut of the population of 348 patients included in the Spanish registry between 1993 and June of 2009, 303 patients with phenotype Pi ZZ were identified. Of these, 194 (64%) were men. The mean age was 54.9 (SD=14 years), and the mean age at the time of diagnosis was 44.1 (SD=14.7 years). A total of 101 patients (33.3%) were never-smokers. The majority (68.8%) were index cases (diagnosed after a study due to lung pathology) and 59 (19.6%) were detected in a family study. All patients showed a mean FEV1 of 1.91L (SD=1.1L) or 63.3% (SD=31%) of the predicted FEV1. The prevalence of significant comorbidity was very low, affecting only 16 cases (5.3%), of which 11 referred cardiac comorbidity (3.6%).
A total of 109 patients (36%) had received substitutive treatment at some time of the follow-up period. Thirty-three patients (10.9%) died during this period, with a mean age at the time of death of 59.2 (SD=10.1 years). The baseline characteristics of the population are presented in Table 1.
Baseline Characteristics of the Patients According to Follow-up.
| With Follow-up (No.=117) | Without Follow-up (No.=186) | Total (No.=303) | P | |
| Sociodemographic data | ||||
| Men, % | 54.7 | 69.9 | 64 | .01 |
| Age, mean (SD) | 55 (13.3) | 55.2 (14.4) | 55 (14) | .58 |
| BMI (kg/m2), mean (SD) | 24.9 (6.4) | 25.1 (4) | 25 (4.1) | .43 |
| Age at the time of diagnosis, mean (SD) | 43 (14) | 45 (15) | 44 (14.7) | .28 |
| Tobacco habit, % | ||||
| Active smokers | 7.7 | 5.4 | 6.3 | |
| Ex-smokers | 61.5 | 59.7 | 60.4 | .59 |
| Never-smokers | 30.8 | 34.9 | 33.3 | |
| Pack-years, mean (SD) | 41.6 (20.8) | 38.9 (21.3) | 40 (21.1) | .56 |
| Reason for AAT determination | ||||
| Lung disease | 67.5 | 69.6 | 68.8 | .017 |
| Family screening | 23.9 | 16.8 | 19.6 | |
| Main pneumopathy | ||||
| Emphysema | 77.8 | 64 | 69.3 | .003 |
| Bronchiectasis | 33.3 | 22.6 | 26.7 | .007 |
| Asthma | 17.1 | 14.5 | 15.5 | .23 |
| Main symptom | ||||
| Dyspnea on exertion | 67.3 | 66.7 | 66.9 | .58 |
| Cough | 14.9 | 16.7 | 15.9 | .63 |
| History of pneumonia | 24.8 | 21 | 22.4 | .16 |
| Treatment, % | ||||
| Bronchodilator | 81.2 | 58.5 | 75.6 | .05 |
| Oxygen therapy | 11.1 | 14.5 | 13.2 | .21 |
| Substitutive treatment | 42.7 | 31.7 | 36 | .05 |
| Lung transplantation | 0.9 | 3.8 | 2.6 | .15 |
| Liver transplantation | 0 | 1.1 | 0.7 | .13 |
| Lung function, mean (SD) | ||||
| Pre-bronchodilator | ||||
| FVC, l | 3.21 (1.22) | 3.33 (1.27) | 3.28 (1.25) | .49 |
| FVC % | 79.7 (22) | 78.9 (24.2) | 79.3 (23.3) | .81 |
| FEV1, l | 1.75 (0.97) | 2 (1.12) | 1.91 (1.1) | .13 |
| FEV1% | 60.4 (30.8) | 64.9 (31.1) | 63.3 (31) | .23 |
| FEV1/FVC | 57.4 (24) | 64.8 (23) | 61.8 (24) | .03 |
| Post-bronchodilator | ||||
| FVC, l | 3.22 (1.21) | 3.35 (1.26) | 3.3 (1.24) | .41 |
| FVC % | 79.2 (22.6) | 79.7 (24.5) | 79.5 (23.7) | .93 |
| FEV1, l | 1.77 (0.99) | 2.07 (1.18) | 1.96 (1.12) | .04 |
| FEV1% | 60.6 (31) | 67.1 (31.5) | 64.6 (31.4) | .08 |
| FEV1/FVC | 57.2 (24) | 64.7 (21) | 61.5 (23) | .01 |
| Exitus, % | 9.4 | 11.8 | 10.9 | .71 |
| Age at the time of death, mean (SD) | 62.9 (9.3) | 57.3 (10.2) | 59.2 (10.1) | .51 |
AAT: alpha-1 antitrypsin; BMI: body mass index; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity.
We obtained 2 or more FEV1 determinations in a sample of 117 patients (38.6% of the total). Table 1 shows the distinct demographic and clinical characteristics between the patients with and without functional follow-up data. Few variables showed significant differences between patients with or without follow-up, even their pre-bronchodilator baseline FEV1 (in % predicted) (60.4% [SD=30.8%] compared to 64.9% [SD=31.1%]; P=.23, respectively) was not significantly different. The patients with spirometric follow-up, on the other hand, showed a greater probability of having been diagnosed by family screening (23.9% compared with 16.8%; P=.017) and they experienced a greater prevalence of emphysema and bronchiectasis compared with the patients without spirometric follow-up.
Substitutive TreatmentOf the 117 patients with spirometric data, 50 (42.7%) had received substitutive treatment at some time of their evolution. Table 2 summarizes the characteristics of the patients with and without substitutive treatment. The patients in substitutive treatment were more frequently men, also had a higher frequency of lung disease, particularly emphysema, and showed a greater prevalence of exertional dyspnea. Likewise, we observed a higher incidence of pneumonia, a greater use of bronchodilators and other respiratory medications, and a greater probability for needing home oxygen therapy.
Patients With Follow-up: Comparison According to the Prescription of Substitutive Treatment.
| Substitutive Treatment (No.=50) | Without Substitutive Treatment (No.=67) | P | |
| Sociodemographic data | |||
| Men, % | 64 | 47.8 | .08 |
| Age, mean (SD) | 58.3 (10.4) | 52.6 (14.5) | .01 |
| BMI (kg/m2), mean (SD) | 25.1 (4.7) | 24.8 (4) | .88 |
| Age at the moment of diagnosis, mean (SD) | 45.7 (9.4) | 40.2 (16.5) | .2 |
| Tobacco habit, % | |||
| Active smokers | 4 | 10.4 | |
| Ex-smokers | 70 | 55.2 | .19 |
| Never-smokers | 26 | 34.3 | |
| Pack-years, mean (SD) | 44.9 (23.2) | 38.3 (17.6) | .3 |
| Reason for the AAT determination | |||
| Lung disease | 78 | 59.7 | .08 |
| Family screening | 18 | 28.4 | |
| Main pneumopathy | |||
| Emphysema | 92 | 67.2 | .001 |
| Bronchiectasis | 44 | 25.4 | .07 |
| Asthma | 20 | 14.9 | .77 |
| Main symptoms | |||
| Dyspnea on exertion | 72.9 | 62.3 | |
| Cough | 12.5 | 17 | .04 |
| Pneumonia | 34 | 17.9 | |
| Treatment, % | |||
| Bronchodilators | 98 | 68.7 | <.001 |
| Oxygen therapy | 18 | 6 | .04 |
| Substitutive treatment | 100 | 0 | |
| Lung transplantation | 0 | 1.5 | .34 |
| Lung function, mean (SD) | |||
| Pre-bronchodilator | |||
| FVC, l | 2.7 (1.04) | 3.55 (1.2) | .002 |
| FVC % | 67.3 (17.2) | 88.1 (21) | <.001 |
| FEV1, l | 1.22 (0.48) | 2.13 (1.06) | <.001 |
| FEV1% | 43.5 (18.8) | 72.2 (32.1) | <.001 |
| FEV1/FVC | 49 (23) | 63 (23) | .008 |
| Post-bronchodilator | |||
| FVC, l | 2.67 (1.01) | 3.63 (1.19) | <.001 |
| FVC % | 66.2 (18.3) | 89 (20.7) | <.001 |
| FEV1, l | 1.24 (0.51) | 2.18 (1.8) | <.001 |
| FEV1% | 43.9 (19.3) | 73.5 (32.1) | <.001 |
| FEV1/FVC | 50 (23) | 62 (23) | .009 |
| Follow-up | |||
| Number of years, mean (SD) | 5.4 (4.2) | 4.2 (3) | .26 |
| Number of visits registered during follow-up, mean (SD) | 3.1 (2.7) | 3.2 (2.4) | .55 |
| Exitus, % | 10 | 9 | |
| Decline in FEV1 (ml/year), mean (SD) | |||
| Pre-bronchodilator | −12.4 (154.7) | +6.4 (163.4) | .72 |
| Post-bronchodilator | −14.4 (111.8) | −1.2 (181.7) | .61 |
AAT: alpha-1 antitrypsin; BMI: body mass index; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity.
The lung function was worse in the patients who received substitutive treatment, with a mean FEV1(%) of 43.5% (SD=18.8%), in comparison with the value observed in those who did not receive substitutive treatment, which was 72.2% (SD=32.1%) (P<.001). No statistically significant differences were detected between the two groups regarding the rate of decline in FEV1, both pre- as well as post-bronchodilator (Table 2).
Rate of Decline in FEV1Out of the 117 patients with follow-up spirometries, 16 were excluded from the analysis of the main variable, either because their determinations were not spaced by at least 6 months or because there was no date available for their determinations. Therefore, this analysis was carried out in 101 patients (33.3% of the total of the population and 83.3% of those with follow-up).
Mean follow-up time was 4.7 years (SD=3.6 years) and the average number of FEV1 measurements per patient was 3.1 (SD=2.5). The mean change observed in FEV1 was +6.9ml/year, with a wide variability (SD=154ml/year).
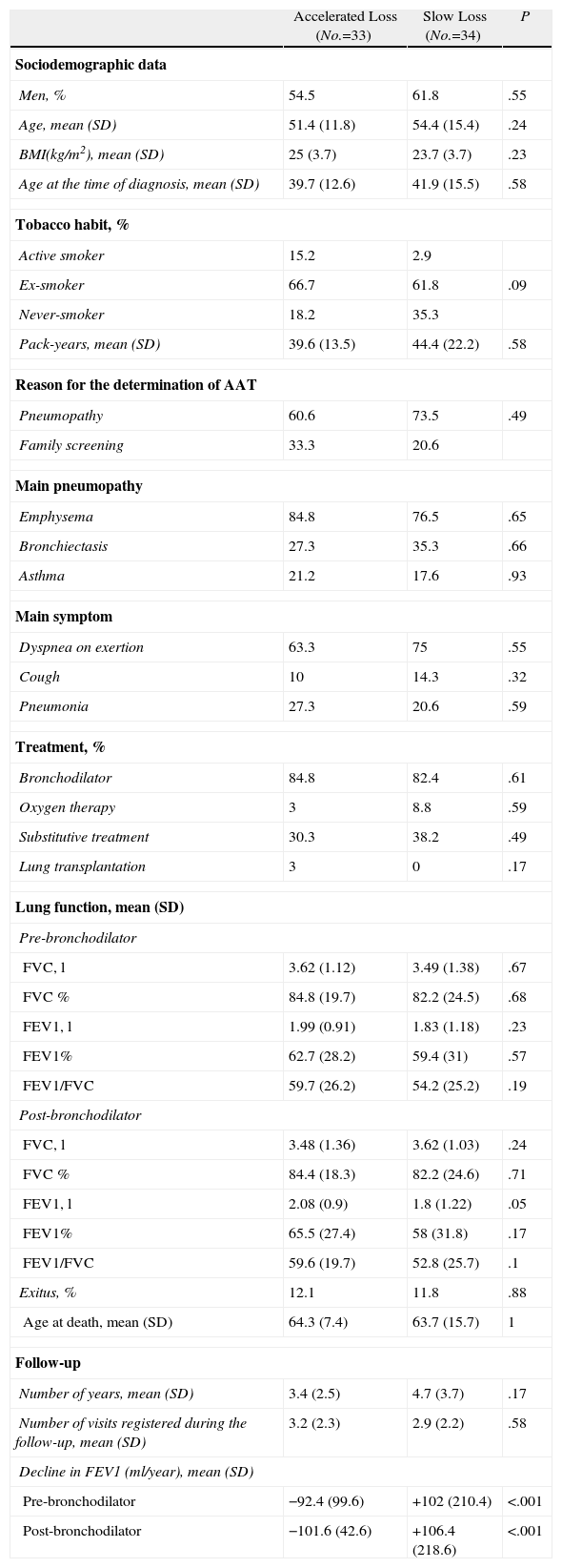
For the analysis of the factors associated with a rate of rapid decline in FEV1, the population was divided into 3 tertiles according to the rate of decline in post-bronchodilator FEV1. The first tertile, or “fast decliners”, experienced a mean rate of descent in FEV1 of −101.6ml/year (SD=42.6ml/year), the third tertile, or “slow decliners”, experienced a positive rate of descent in FEV1 (gain) of +106.4ml/year (SD=218ml/year), and the middle tertile showed a mean rate of decline in FEV1 of −28.4ml/year (SD=14ml/year). In order to analyze the characteristics of the patients with different rates of deterioration in lung function, we compared the characteristics of the fast and slow decliner patients (first and third tertile) (Table 3).
Patients With Registered Follow-ups: Comparison Between the Patients With Accelerated Loss and With Slow Loss.
| Accelerated Loss (No.=33) | Slow Loss (No.=34) | P | |
| Sociodemographic data | |||
| Men, % | 54.5 | 61.8 | .55 |
| Age, mean (SD) | 51.4 (11.8) | 54.4 (15.4) | .24 |
| BMI(kg/m2), mean (SD) | 25 (3.7) | 23.7 (3.7) | .23 |
| Age at the time of diagnosis, mean (SD) | 39.7 (12.6) | 41.9 (15.5) | .58 |
| Tobacco habit, % | |||
| Active smoker | 15.2 | 2.9 | |
| Ex-smoker | 66.7 | 61.8 | .09 |
| Never-smoker | 18.2 | 35.3 | |
| Pack-years, mean (SD) | 39.6 (13.5) | 44.4 (22.2) | .58 |
| Reason for the determination of AAT | |||
| Pneumopathy | 60.6 | 73.5 | .49 |
| Family screening | 33.3 | 20.6 | |
| Main pneumopathy | |||
| Emphysema | 84.8 | 76.5 | .65 |
| Bronchiectasis | 27.3 | 35.3 | .66 |
| Asthma | 21.2 | 17.6 | .93 |
| Main symptom | |||
| Dyspnea on exertion | 63.3 | 75 | .55 |
| Cough | 10 | 14.3 | .32 |
| Pneumonia | 27.3 | 20.6 | .59 |
| Treatment, % | |||
| Bronchodilator | 84.8 | 82.4 | .61 |
| Oxygen therapy | 3 | 8.8 | .59 |
| Substitutive treatment | 30.3 | 38.2 | .49 |
| Lung transplantation | 3 | 0 | .17 |
| Lung function, mean (SD) | |||
| Pre-bronchodilator | |||
| FVC, l | 3.62 (1.12) | 3.49 (1.38) | .67 |
| FVC % | 84.8 (19.7) | 82.2 (24.5) | .68 |
| FEV1, l | 1.99 (0.91) | 1.83 (1.18) | .23 |
| FEV1% | 62.7 (28.2) | 59.4 (31) | .57 |
| FEV1/FVC | 59.7 (26.2) | 54.2 (25.2) | .19 |
| Post-bronchodilator | |||
| FVC, l | 3.48 (1.36) | 3.62 (1.03) | .24 |
| FVC % | 84.4 (18.3) | 82.2 (24.6) | .71 |
| FEV1, l | 2.08 (0.9) | 1.8 (1.22) | .05 |
| FEV1% | 65.5 (27.4) | 58 (31.8) | .17 |
| FEV1/FVC | 59.6 (19.7) | 52.8 (25.7) | .1 |
| Exitus, % | 12.1 | 11.8 | .88 |
| Age at death, mean (SD) | 64.3 (7.4) | 63.7 (15.7) | 1 |
| Follow-up | |||
| Number of years, mean (SD) | 3.4 (2.5) | 4.7 (3.7) | .17 |
| Number of visits registered during the follow-up, mean (SD) | 3.2 (2.3) | 2.9 (2.2) | .58 |
| Decline in FEV1 (ml/year), mean (SD) | |||
| Pre-bronchodilator | −92.4 (99.6) | +102 (210.4) | <.001 |
| Post-bronchodilator | −101.6 (42.6) | +106.4 (218.6) | <.001 |
AAT: alfa-1 antitrypsin; BMI: body mass index; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity.
Few differences were observed between the patients who experienced a “rapid fall” and those who showed a “slow fall”. In the first group, there were less non-smokers (18.2% compared to 35.3%; P=.09) and the age of onset of the symptoms was earlier (31.2 compared with 38.6 years; P=.034). Baseline lung function, represented by mean pre-bronchodilator FEV1 (%) was similar in both groups: “rapid fall”, 62.7% (SD=28.2%) compared with “slow fall”, 59.4% (SD=31%); P=.57).
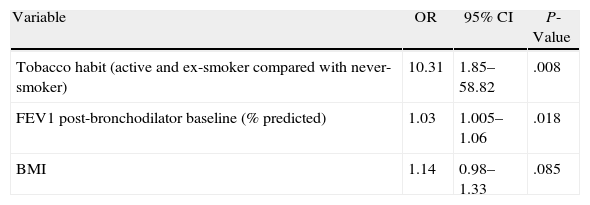
In the stepwise logistic regression model, with the dependent variable “rapid fall”, the following variables were significantly and independently associated with a faster rate of decline in FEV1: (a) being a smoker or ex-smoker, compared with being a non-smoker (OR=10.31; 95% confidence interval (CI)=1.8–58.8; P=.008); and (b) having a greater baseline post-bronchodilator FEV1 (%) (OR=1.03; 95% CI=1.005–1.06; P=.018). In addition, there was an observed tendency towards an association between body mass index (BMI) and experiencing a greater rate of loss of lung function, which did not reach statistical significance (OR=1.14; 95% CI=0.98–1.33; P=.085) (Table 4). In order to validate the model, we used the Hosmer–Lemeshow test, whose result confirmed the goodness of fit with a P-value of 0.29 (if P>.05, the null hypothesis is accepted).
Identification of Variables Associated With the Decline of Post-bronchodilator FEV1 By Means of Stepwise Logistic Regression Analysis.
| Variable | OR | 95% CI | P-Value |
| Tobacco habit (active and ex-smoker compared with never-smoker) | 10.31 | 1.85–58.82 | .008 |
| FEV1 post-bronchodilator baseline (% predicted) | 1.03 | 1.005–1.06 | .018 |
| BMI | 1.14 | 0.98–1.33 | .085 |
BMI: body mass index; FEV1: forced expiratory volume in 1s; OR: odds ratio; CI: confidence interval.
This retrospective study based on data included in the REDAAT has shown that there is a great variability in the annual rate of decline in the FEV1 of patients with AATD and that the risk factors associated with an accelerated rate of decline in FEV1 were a history of smoking, better lung function at the beginning of follow-up and a low BMI. When we compared the rate of descent in the FEV1 of patients who had either received at one time or were receiving substitutive treatment with those who had never received it, no statistically significant differences were observed in the annual rate of fall in FEV1.
It still has not been possible to recruit enough patients with AATD in order to carry out a randomized study with sufficient statistical power in order to demonstrate the efficacy of the substitutive treatment in reducing the speed of the deterioration in lung function. With the intention of overcoming the methodological difficulties due to the low prevalence of AATD and the variable probability of developing related symptoms despite having AATD, a series of registries have been created in order to compile and analyze the clinical data of individuals with AATD, adjusted to real life.8,25,26 These registries offer the opportunity to evaluate large cohorts of individuals in an almost experimental manner that is closer to real-life conditions over long expanses of time. Their inclusion criteria are less strict than those of clinical assays, and there is no requisite of the prescription of a certain treatment for the participation of the individuals included. As a consequence, the observational studies with patients recruited from registries have allowed us to research the impact of substitutive treatment on the speed of the fall in FEV1 and on survival.7,17,27,28
The AATD registries can have different objectives and examine inhomogeneous populations with this genetic alteration. For example, the NHLBI Alpha-1-Antitrypsin Deficiency Registry was founded in 1988 with the objective of characterizing the clinical course of this disease in 1000 individuals with AATD using the data collected during 7 years in centers in the United States and Canada.25 The declining rates in lung function and survival were key aspects evaluated in this registry. A second registry of larger dimensions, AOF-RNR, was created in 2000 by the initiative of a foundation of affected patients (Alpha One Foundation, Miami, FL, United States) with the aim to describe the largest population possible of deficient individuals in order to facilitate future clinical studies.26 The phenotype of the patients included in the AOF-RNR was reported by the patients themselves and, consequently, the registry contains patients with unconfirmed genotypes. The AIR (Alpha-1 International Registry) was founded in 1998 in response to the recommendations of the World Health Organization.8 The objective of this initiative was to create a common, centralized database in order to obtain knowledge about AATD by means of the data compiled of patients from more than 20 participating countries. With 2627 subjects recruited before 2006, AIR is the most extensive and has the largest volume of clinical and demographic data of individuals with AATD.8
The Spanish registry of patients with AATD was founded in 1993 with the following objectives: (a) to learn about the characteristics and frequency of AATD in Spain; (b) to establish guidelines adapted to our setting about the treatment and follow-up of patients with the deficiency; (c) to offer information to the doctors who treat these patients throughout Spain; (d) to increase the knowledge and interest in this disease and try to reduce the underdiagnosis and the delay in diagnosis; and (e) to offer technical support for the determination of the phenotype Pi and, if necessary, the genotype in individuals suspected of being deficient.19,20,29
The population analyzed in this study included only patients with severe AATD. Differences and similarities were observed when we compared them with the baseline characteristics reported in the AIR, NHLBI and AOF-RNR registries. In the Spanish population, 64% were men compared with 56%, which is the mean in the remainder of the registries. In the same way, the mean age of the patients of the Spanish population was slightly older compared with other registries (55 years of age vs 46–50, respectively). The proportion of patients identified by family study was similar in the NHLBI, AIR and in the Spanish registry.8 In the latter, 6% were active smokers, unlike the AIR, NHLBI and AOF-RNR registries, with a reported 10%, 8% and 2%, respectively. The low rate of tobacco habit observed in the AOF-RNR registry could reflect a greater degree of consciousness about the risks associated with smoking in the patients with AATD.8 The mean FEV1(%) was lower in the population of NHLBI in comparison with the Spanish population (47% vs 63%), and the majority of patients of the NHLBI registry either were receiving or had previously received substitutive treatment (66% vs 36%, respectively).7,30
The method used to calculate the rate of fall in lung function is based on the measurement of the two extreme values in the follow-up. This method has been used in a clinical assay with intravenous AAT together with a linear regression method, and both offered similar results,24 therefore we have adopted the method described (end-point analysis). The findings found in our logistic regression analysis–that being a smoker and having a higher baseline post-bronchodilator FEV1 would be significantly and independently associated with a more rapid rate of decline in FEV1–are important observations of this study. The results agree with the findings of previous studies. Both in the NHLBI7 registry and in the Swedish AATD registry,31 we observed a faster deterioration of lung function in active smokers than in ex-smokers and never-smokers, while Dowson et al.32 have demonstrated that the deterioration of FEV1 is greater in individuals with a poorer post-bronchodilator FEV1. In addition, Dawkins et al. observed a greater degree of descent in lung function in individuals with moderate-severe disease compared with those with very severe affectation.33
The results, likewise, showed a tendency towards the association between a low BMI and the probability of experimenting a more rapid rate of decline of the FEV1.33 Consistently, previous studies have demonstrated that a low BMI is an independent predictor for mortality in patients with AATD.34
Several studies have shown that substitutive treatment can slow the rate of decline of lung function,7,17,27,28 reduce the loss of lung tissue and the progression of emphysema,24 and increase survival in patients with AATD.7 In the Spanish registry, however, no significant difference was observed in the rate of decline of FEV1 according to whether or not the patients received substitutive treatment. Although this could be an unexpected finding, it is important to admit that both groups had considerably different baseline characteristics. The patients that received substitutive treatment were more frequently men with lung disease and with greater exertional dyspnea than those who did not receive substitutive treatment. In addition, the patients with substitutive treatment had a greater incidence of pneumonia, greater use of medication for their respiratory disease and greater use of chronic home oxygen therapy. Also, the mean FEV1 of the patients in substitutive treatment was significantly less than that of the group who did not receive substitutive treatment. The differences between the groups in baseline conditions exclude the possibility of a reliable evaluation of the effects of the substitutive treatment in the rate of decline in FEV1, and it is another example of the challenges that are faced when interpreting data derived from observational studies such as registries. For example, the decision of the prescription is made by each doctor depending on the severity and the prognosis of each patient, which usually results in an indication bias by assigning the treatment preferentially in more severe patients or with more risk factors for a poor prognosis. Likewise, it is not possible to analyze the influence of the respiratory medication on the fall in FEV1 as there is a bias in indication, by prescribing more medication to the more severe patients or those who present a poorer clinical evolution. In this study, it was not possible to evaluate the influence of other treatment strategies like respiratory rehabilitation as this variable was not included in the original database of the international registry.
Other factors, such as respiratory infections and age, can modulate the deterioration of the lung function over time, although with a lower intensity. The role that these factors play is not as important as smoking, and it is necessary to study large cohorts of patients in order to observe its possible impact.
In addition to the limitations derived from the different characteristics of the patients with or without substitutive treatment, there are other considerations that should be kept in mind when interpreting the findings presented here. First of all, the Spanish registry is not a population registry and may not be representative of all the individuals with this genetic disorder. The registry compiles a high percentage of patients diagnosed with AATD due to presenting lung disease, mostly emphysema, but the asymptomatic individuals are underrepresented. It is possible that the symptomatic and asymptomatic individuals experience different rates of deterioration in their lung function, even after controlling for factors such as tobacco habit and the initial values of lung function. Second, only a third of the patients who were initially identified were followed-up and, therefore, were valid for the analysis of the main variable. Differences, such as having poorer baseline lung function, were data that were seen in the group with follow-up and apt for the calculation of the fall in FEV1 in comparison with the group without follow-up. Therefore, it is not assumed that the results that are presented here can be applied in general to all the patients of the registry.
Despite the limitations, the findings reported in this analysis provide valuable information about the natural history of the disease and suggest various parameters with a potential predictive value in the detection of the rapid deterioration of the lung function in affected individuals.
ConclusionsThe rate of deterioration in lung function in patients with AATD varies ostensibly. The Spanish registry has provided data that show that smoking and having a more preserved lung function are significantly related with an accelerated decline in FEV1. This suggests that the youngest patients, with normal or hardly deteriorated lung function, should undergo a more intense follow-up of their evolution together with emphatic anti-tobacco advice.
The cooperation of multiple researchers in a national registry is not an easy task, and the prospective collection of data requires quite a lot of motivation. Centralized registries, such as those in Britain or the Netherlands, are more appropriate for longitudinal data collection. New strategies should be started to encourage physicians to include and periodically update the follow-up data of their patients in the databases of the registries.
Conflict of InterestsThe authors declare having no conflict of interests related to this manuscript.
The present study has been financed with a research grant from Talecris Biotherapeutics GmbH (Frankfurt am Main, Germany).
This study has been financed by Talecris Biotherapeutics GmbH, Germany. In addition, Martin Kenig, D.Phil, from PAREXEL, has assisted in the writing of the manuscript, under the direction of the authors, and with the financial support from Talecris Biotherapeutics, Inc.
The authors would like to thank the physicians participating in REDAAT because, without the data they have contributed, this study would not have been possible. We would also like to thank Dr. Rosendo Jardí and Dr. Francisco Rodríguez-Frías (Biochemistry Department, Hospital Universitario Vall d’Hebron, Barcelona) for their collaboration in determining the phenotypes and genotypes of the cases included in the REDAAT, and Montse Pérez for her help with the statistical analysis.
REDAAT Coordinators: Francisco Casas-Maldonado (Granada), Maria Teresa Martínez (Madrid); Administration: Beatriz Lara (Lleida); Advisory committee: Ignacio Blanco (Langreo), Ana Bustamante (Torrelavega), Sergio Cadenas (León), Maria Teresa Martínez (Madrid), Lourdes Lázaro (Burgos), María Torres (La Coruña), José María Hernández (La Palma), Rafael Vidal (Barcelona), Marc Miravitlles (Barcelona), Alberto Herrejón (Castellón), M. Jesús Cabero (Santander), Lino Alvarez (Santander), Gloria García (Madrid), Cristóbal Esteban (Galdakano), Adolfo Doménech (Seville); Central laboratory of the Registry: Rosendo Jardí and Francisco Rodríguez-Frías (Barcelona).
Please cite this article as: Tirado-Conde G, et al. Factores asociados a la evolución de la función pulmonar en pacientes con déficit de alfa-1-antitripsina del registro español. Arch Bronconeumol. 2011. doi:10.1016/j.arbres.2011.06.002.