Lung function reference values are traditionally based on anthropometric factors, such as weight, height, sex, and age. FVC and FEV1 decline with age, while volumes and capacities, such as RV and FRC, increase. TLC, VC, RV, FVC and FEV1 are affected by height, since they are proportional to body size. This means that a tall individual will experience greater decrease in lung volumes as they get older. Some variables, such as FRC and ERV, decline exponentially with an increase in weight, to the extent that tidal volume in morbidly obese patients can be close to that of RV. Men have longer airways than women, causing greater specific resistance in the respiratory tract. The increased work of breathing to increase ventilation among women means that their consumption of oxygen is higher than men under similar conditions of physical intensity. Lung volumes are higher when the subject is standing than in other positions. DLCO is significantly higher in supine positions than in sitting or standing positions, but the difference between sitting and standing positions is not significant. Anthropometric characteristics are insufficient to explain differences in lung function between different ethnic groups, underlining the importance of considering other factors in addition to the conventional anthropometric measurements.
Los valores de referencia de las pruebas de función pulmonar están basados históricamente en factores antropométricos como el peso, la altura, el género y la edad. La FVC y el FEV1 disminuyen con la edad y, en contraposición, volúmenes y capacidades como el RV y la FRC se incrementan. La TLC, CV, RV, FVC y FEV1 se ven afectados por la altura, puesto que son proporcionales al tamaño corporal. Esto significa que un individuo alto sufrirá un mayor decremento de sus volúmenes pulmonares a medida que aumente su edad. Algunas variables decrecen exponencialmente con el incremento del peso, como la FRC y el ERV, de tal forma que los sujetos con obesidad mórbida pueden llegar a alcanzar un volumen corriente cercano al RV. Los hombres poseen vías aéreas de conducción más largas que las mujeres, dando lugar a una mayor resistencia específica de las vías respiratorias. El mayor trabajo respiratorio en mujeres para aumentar la ventilación provoca que, en condiciones con la misma intensidad física, el consumo de oxígeno sea más alto que en hombres. En posición vertical los volúmenes pulmonares son más altos que en el resto de las posturas. La DLCO es significativamente mayor en posiciones supinas que en posición sentada y vertical, no existiendo diferencias significativas en posición sentada y de pie. Las características antropométricas no son suficientes para explicar las diferencias existentes en la función pulmonar entre diferentes etnias y ponen de manifiesto la importancia de considerar otros factores adicionales a los clásicos antropométricos para su medición.
Lung function tests (LFTs) are a combination of studies conducted in clinical practice to determine lung capacity and possible deterioration of the mechanical function of the lungs, respiratory muscles, and chest wall. These tests are useful for confirming the possible existence and severity of lung diseases, and also for evaluating respiratory response to possible therapeutic interventions.1
Reference values for LFTs in clinical practice are often difficult to estimate. In clinical guidelines, the interpretation of lung function has historically been based on the most important anthropometric factors, including weight, height, sex, and age.2 However, as knowledge in this area has grown, other factors have gained particular relevance. The most important of these are physical parameters (circadian rhythms,3 menstrual cycle,4 chest diameter,5 trachea size),6 social and healthcare considerations (educational level,7 socioeconomic status,8 workplace exposures),9 environmental factors (air pollution,10 climatic conditions,11 natural disasters,12 altitude),13 race or ethnic group,14 lifestyle (nutrition,15 level of physical activity,16 smoking),17 diseases (diabetes,18 muscle or hormone disorders),19 physical position,20 genetic factors,21 war situations (military conflicts,22 terrorist attacks),23 and even influencing factors occurring during childhood24 or pregnancy.25
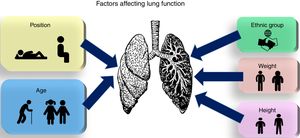
Although a more or less extensive bibliography is available on how each of the factors mentioned above affects lung morphology and function, to the best of our knowledge, there are no recent reviews that discuss and integrate all these elements into a single comprehensive source. This review aims to focus on anthropometric factors, physical position, and race and ethnic groups, to give an overview of how these factors affect lung function (Fig. 1).
Materials and MethodsThis study was based primarily on a systematic review of the existing literature on factors affecting lung function. To this end, we have used several free-access search engines, including PubMed, Lilacs, SciELO, GoPEDro, Google Scholar, and Scopus databases. Reviews, observational studies, clinical trials, and mathematical models particularly from the last five years were selected, although some older publications that were of particular relevance to the topic were also considered.
The literature search was based on various key words used independently or in combination with the use of OR and AND operators. The search terms included: “lung function testing”, “pulmonary function tests”, “pulmonary function”, “pulmonary impairment”, “spirometry”, “plethysmograph”, “lung volumes”, “diffusing capacity”, “arterial blood gases”, “chronic obstructive pulmonary disease” and “lung function values”, as well as all and each of the spirometric variables analyzed in this study and the correlations between these, and ethnic groups (primarily Caucasian, African American, and Asian) and each factor affecting lung function.
ResultsEach of the factors that have the most effect on lung function and those of most interest for this study will be examined in detail in this section.
AgeAge has historically been one of the major factors in the evaluation of lung function. Pulmonary maturity is reached at about 20–25 years of age,26 after which lung function progressively begins to decline.27
The variables most affected are forced vital capacity (FVC) and forced expiratory volume in 1second (FEV1); these decline with age28 due to decreased compliance of the chest wall, loss of expiratory muscle strength, and the growing tendency of the smaller airways to close during forced expiration.29 Specifically, FEV1 declines by around 20ml/year between the ages of 25 and 39 years, a rate which gradually increases until it reaches 35ml/year after the age of 65.30 The FEV1/FVC ratio also decreases with age, with the steepest decline occurring between the ages of 3 and 10 years, due to the marked increase of FVC compared to FEV1 that occurs during that period. This trend is temporarily reversed during childhood, when the FEV1/FVC ratio rises slightly until the age of 16, after which the decline is continuous. This decline is presumably due to the gradual loss of lung elasticity. In contrast, volumes and capacities, such as residual volume (RV) and functional residual capacity (FRC) increase, while vital capacity (VC) and inspiratory capacity (IC) fall as a result of airway closure, progressive hardening of lung tissue, and loss of elastic recoil pressure.31 Finally, total lung capacity (TLC) generally remains constant in the absence of disease.30,32
Gas exchange tends to decrease with age due to the loss of alveolar surface and reduced blood volume.33 During childhood, PAO2 and PACO2 do not change significantly, but PaO2 increases gradually during adolescence. Ventilatory response to hypercapnia and hypoxia is highest in early childhood and fall gradually until adulthood.34 Specifically, the decline in carbon monoxide diffusion in the lung (DLCO) is around 0.2mlCO/min/mmHg/year.30,35 This also causes a progressive decrease in PaO2 from 95mmHg at the age of 20, to 75mmHg at the age of 70, although PaCO2 remains unchanged while ventilation increases.
Very few studies in the literature have attempted to use longitudinal studies to address the problem of the impact of age on exercise capacity.36 Maximal oxygen consumption (VO2max) reduces with age, although this decline is considerably delayed in physically active subjects. The decline in exercise capacity becomes more marked after the age of 60, when a normally healthy individual might begin to experience frequent episodes of dyspnea during physical exercise.27
More specific studies focusing on the deterioration of the respiratory system with age, which also have some clinical relevance, have explored factors in children with low birth weight,37 menopausal women,38 or the role of cellular senescence in the aging of the lungs.39 Studies have also been published that examine exposures in early life that impact lung function in adulthood.40
HeightSeveral parameters, such as TLC, VC, RV, FVC and FEV1, are affected by height, since they are proportional to body size.41 This means that in tall individuals, who accordingly have greater lung capacity, lung volume will decrease at a greater rate to shorter individuals as they grow older.42 Peak expiratory flow (PEF) is also greater in taller individuals, and predictive equations, such as the Wright scale,43 the IN1382, or the NHANESIII,44 are widely used in clinical practice. Finally, DLCO values in gas exchange have also been shown to increase with height.45
WeightIn general, TLC decreases as the body mass index (BMI) increases, although the decline is insignificant, even in morbid obesity.46 This preservation of both TLC and VC is due to the compensatory effect of IC, which increases with obesity. This increase in IC and the corresponding decrease in RV are due to the displacement of the diaphragm toward the chest cavity as a result of the mechanical pressure exerted by excess fat. As a consequence, tidal volume (VT) at rest and during physical exercise tends to decrease, producing a smaller expiratory flow.47
Other variables, such as FRC and expiratory reserve volume (ERV), have been seen to decline exponentially with an increase in BMI, to the extent that VT in morbidly obese patients can approach that of RV.14 FRC can be become so low that it is exceeded by closing capacity. This phenomenon has clinical implications, and may cause the lungs to collapse and atelectasis to develop in the lower lung zones, leading to a non-uniform distribution characterized by gas exchange taking place primarily in the upper zones.48
With regard to inspiratory/expiratory flows, PEF falls as BMI increases. There are discrepancies in the literature regarding FEV1 and FVC. Some authors claim that obesity generally does not cause FEV1 and FVC to fall, unless patients are morbidly obese, so the FEV1/FVC ratio also remains constant.49 However, other studies suggest that obesity can cause airway limitation, causing a parallel reduction in FEV1 and FVC, thus preserving the FEV1/FVC ratio.50 Abdominal obesity is generally associated with reduced FEV1 and FVC in women and in certain age groups.51
Studies published on DLCO also present conflicting results. Some authors have found that DLCO values fall as BMI increases, while others report that they rise or are maintained.52,53 One reason for this discrepancy could be that DLCO depends on various factors not exclusively associated with lung function, such as hemoglobin levels or KCO. An increase in BMI generally causes a deterioration in alveolar gas exchange, causing PaO2 to fall.54
With regard to weight-related respiratory implications during exercise, VT is lower and respiratory rates higher in obese individuals than in non-obese individuals.55 Moreover, excess fat in the chest wall causes VO2 for each breath to increase, intensifying the ventilatory demand that is manifested by greater metabolic cost, increasing VO2 and carbon dioxide production (VCO2).47,56
Finally, thinness also has a relative impact on lung function. Most spirometric values fall when weight is low, resulting in decreased ventilatory capacity. Thus, underweight individuals have low FVC and FEV1, and respiratory muscle deficit. Other spirometric values that decrease are VC and ERV.57 These effects are also observed in children and infants with low birth weight.58
Some contradictions observed in studies that focus on the impact of weight on lung function may be due to their exclusive use of the concept of BMI as a measure of obesity. This leads other factors that may be of significance being ignored, such as central obesity (mainly in men), peripheral adiposity (more common in women),59 skin fold thickness, waist circumference, and waist-to-hip ratio.60
SexStandard morphometric methods confirm that among individuals of the same weight and height, men have larger lungs than women, and consequently, a larger number of bronchi, greater alveolar surface area, and a wider airway caliber.61
The number and size of the alveoli increases during postnatal lung development. While the female lung is smaller than the male lung and has fewer bronchioles, the number of alveoli per unit of surface area is the same in both boys and girls. Then, during adolescence, a phenomenon called dysanapsis, or differential growth between airway size and lung size, occurs. Thus, in females, airway growth is proportional to lung tissue, while in males, disproportionate airway growth results in a much smaller number of alveoli compared to the number of airways. Males, then, have longer conducting airways than females, putting them at a clear disadvantage during expiration at this stage of their lives, and producing greater specific airway resistance and lower rates of PEF.62 Even in lungs of the same size, males still have larger airways than females. However, when growth is complete and lung maturity is approaching, the situation normalizes, and FRC, VC, TLC, RV, and peak flows are higher in males than in females. With regard to elastic recoil, chest wall characteristics, and lung compliance, differences between men and women do not appear to be significant, although the trachea caliber is smaller in women.63,64
Men also have higher airflow values than women, although the FEV1/FVC ratio remains the same.65 Ventilation, peak inspiratory pressure, and expiratory flows are also higher in men than in women. Overall, FEV1 decreases with age, but the decline is more rapid in men than in women, since respiratory muscle strength decreases more dramatically in men than in women.26,30
With regard to gas diffusion, DLCO is higher in men than in women, and falls at a rate of approximately 0.2mlCO/min/mmHg/year in men and 0.15mlCO/min/mmHg/year in women.30 This difference may be due to the influence of estrogen in maintaining the integrity of the blood vessels in women. The menstrual cycle has also been shown to modify gas diffusion mechanisms, characterized by a DLCO peak just before the start of menstruation, followed by a rapid decline that troughs on day 3 of the cycle.4 These changes are thought to be due to blood capillary volumes.66 No significant differences between sexes have been observed in the DLCO/alveolar volume (VA) ratio.
With regard to physical exercise, the smaller airway caliber and lung volumes in women produce lower peak expiratory flows. Women, thus, have a much lower capacity than men for increasing ventilation during exercise. However, neither men nor women reach their peak effective ventilation during highly strenuous physical exercise, although women come closer to this value than men. This increased work of breathing to increase ventilation among women means that their consumption of oxygen is higher than in men under similar conditions of physical intensity, and this negatively impacts on general performance during exercise.67 Moreover, women are more prone to arterial desaturation during intense exercise, so their performance is even more limited.68,69 In general, VO2max falls at a rate of 32ml/min/year in men, and 14ml/min/year in women after the age of 20–30 years.30 In addition, morphological characteristics of the lungs of women can contribute to inadequate hyperventilation and a consequent increase in exercise-induced hypoxemia.70 Finally, DLCO in men during physical exercise is almost double that of women, due to increased pulmonary blood volume (about 40% more).53
PositionLung distensibility is significantly reduced with changes in position from standing upright, sitting down, and lying down on the back, side or front. Lung volumes are higher when the subject is standing than in other positions, due to an increase in the volume of the chest cavity. VC and TLC fall in the supine position compared to the standing position, possibly due to changes in blood flow from the lower limbs to the chest cavity.71 In decubitus positions, VC is higher in supine decubitus than in prone decubitus, while TLC does not change significantly. FRC is also reduced in decubitus positions, more specifically in the supine position because the abdomen pushes the diaphragm toward the chest cavity. As a result, FRC and ERV values are higher when standing than when sitting or lying down, while values in the sitting position are higher than in the supine position. Differences between lying in a prone or a supine decubitus position are not significant,72 so the increase in intra-abdominal pressure leads to an increase in FRC and ERV. VT values are higher in a sitting position than in a supine position. This is because the progressively increased inclination of the trunk determines a reduction of rib cage displacement and ventilation, so VT gradually increases as the back is raised into the upright position.73 However, the highest VT values are achieved in a standing position. A sitting position also results in a decrease in VT.
Some studies have shown that FEV1, FVC, and the FEV1/FVC ratio are higher in a standing position than in a sitting or lying position.74 However, these results differ from other studies that report that FVC and FEV1 values are higher in a sitting position than in a standing or lying position, with no significant differences in forced expiratory flow between 25% and 75% of vital capacity (FEF25–75). Reported values for FVC and FEV1 do appear to be consistently lower in supine and prone positions compared to the sitting position.75 Body position also affects PEF values significantly.76
DLCO is higher in supine positions than in sitting and standing positions.77 However, no significant differences in DLCO are found when subjects are sitting or standing. With regard to differences between the supine and prone positions, some studies have shown a mild reduction in healthy subjects when they change from the supine position to the prone position.78
Race or Ethnic GroupMany of the studies that analyze the effect of race on lung function use the cormic index, defined as the ratio of sitting height to standing height, as a methodological basis for classifying measurements. However, while anthropometric characteristics play a fundamental role, they are not sufficient to explain differences in lung function among different ethnic groups. Skin color also appears to be an inadequate indicator.79,80
The American Thoracic Society (ATS) and the European Respiratory Society (ERS) have published recommendations for obtaining spirometric values using reference equations derived from samples of different races/ethnic groups, whenever possible.81 Standard values are normalized using a “race correction”, also called an “ethnic adjustment”, generally with the use of a scaling factor for all individuals not considered white or Caucasian.82
It can be generally stated that differences exist in lung function values among the main races of the world for whom sufficient data are available,83 namely the following four groups: whites or Caucasians (Europeans, Israelis, Australians, United States Americans, Canadians, Brazilians, Chileans, Mexican Americans, Uruguayans, Venezuelans, Algerians, Tunisians), blacks (African Americans), north-east Asians (Koreans and northern Chinese), and south-east Asians (Thais, Taiwanese, southern Chinese, and Hongkongers).
ConclusionsOur systematic literature review highlights a great interest in the study of factors that affect lung function, including the classic anthropometric determinations. The use of reference standards in clinical practice provides reasonable theoretical values for a large sector of the population, although some factors that may be relevant in certain cases are not taken into account. These include body fat distribution in obese individuals, respiratory system changes during childhood, puberty, and menopause, lifestyle habits, or position of the body during the conduct of the tests. Future research should also focus on the study of differences in lung volume among certain races and ethnic groups in some regions of the world, given the lack of literature in this area. No multivariate studies have been conducted on the different variables analyzed. However, an integrated vision of all of these factors that determine the measurement of lung function must be developed and included in reference tables for use in clinical practice.
Conflict of InterestThe authors state that they have no conflict of interests.
This work was supported in part by the “Instituto de Salud Carlos III” under Grants PI15/00306 and DTS15/00195, and in part by the “Fundación Progreso y Salud”, Government of Andalucía, under Grant PI-0010-2013, PI-0041-2014 and PIN-0394-2017.
Please cite this article as: Talaminos Barroso A, Márquez Martín E, Roa Romero LM, Ortega Ruiz F. Factores que afectan a la función pulmonar: una revisión bibliográfica. Arch Bronconeumol. 2018;54:327–332.