Despite improvements in ventilation support techniques, lung protection strategies and the application of new support treatment, acute respiratory distress syndrome continues to have a high mortality rate. Many strategies and treatments for this syndrome have been investigated over the last few years. However, the only therapeutic measure that has systematically shown to be able to improve survival is that of low volume lung protective ventilation. Thus, using a low tidal volume prevents added lung damage by the same mechanical ventilation that is essential for life support. In this context, the use of extracorporeal lung assist systems is considered as a salvage treatment for use in extreme cases. On the other hand, it could be a potentially useful complementary method for an ultra-protective ventilation strategy, that is, by using even lower tidal volumes. The currently available extracorporeal lung assist systems are described in this article, including high flow systems such as traditional extracorporeal membrane oxygenation, CO2 removal systems (interventional lung assist or iLA, with or without associated centrifugal pumps), and the new low flow and less invasive systems under development. The aim of this review is to update the latest available clinical and experimental data, the indications for these devices in adult respiratory distress syndrome (ARDS), and their potential indications in other clinical situations, such as the bridge to lung transplantation, multiple organ dysfunction syndrome, and COPD.
A pesar de las mejoras de las técnicas de soporte ventilatorio, las estrategias de protección pulmonar y la aplicación de nuevos tratamientos de soporte, el síndrome de distrés respiratorio agudo continúa asociando una alta mortalidad. Durante los últimos años, se ha investigado una extensa cantidad de estrategias y medidas de tratamiento para este síndrome. Sin embargo, la única medida terapéutica que ha demostrado sistemáticamente ser capaz de mejorar la supervivencia es la estrategia de ventilación pulmonar protectora mediante bajos volúmenes. Así, empleando un volumen corriente bajo se evita el daño pulmonar añadido por la propia ventilación mecánica imprescindible para el mantenimiento vital. En este contexto, el empleo de sistemas de asistencia respiratoria extracorpórea se considera un tratamiento de rescate de uso excepcional en casos extremos. Por otro lado, podría ser también un método complementario potencialmente útil para permitir una estrategia de ventilación ultra-protectora, es decir, empleando volúmenes corrientes aún más bajos. En este artículo se describen los sistemas disponibles de asistencia respiratoria extracorpórea incluyendo sistemas de alto flujo, como la membrana de oxigenación extracorpórea tradicional, los sistemas de eliminación de CO2(interventional lung assist o iLA, con o sin bombas centrífugas asociadas), y los nuevos sistemas de bajo flujo y menor invasividad en desarrollo. El objetivo de esta revisión es actualizar los últimos datos experimentales y clínicos disponibles, la indicación de estos dispositivos en el síndrome de distress respiratorio del adulto (SDRA) y sus posibles indicaciones potenciales en otras situaciones clínicas, como el puente a trasplante pulmonar, síndrome de disfunción orgánica múltiple o la EPOC.
Mechanical ventilation is a fundamental tool for critical care of patients with acute respiratory failure. In spite of the efficiency of this life support method, mechanical ventilation is not without its complications. Overdistension and cyclic collapse and reopening of alveolar units damages the alveolar-capillary barrier, hampering gas exchange and lung mechanics. Ventilation causes trauma from pressure, changes in volume, and a local and systemic inflammatory response that contributes to lung damage. This type of lesion is especially relevant in adult respiratory distress syndrome (ARDS). Patients with ARDS typically present with devastating respiratory failure and severe damage to ventilatory mechanics. In 1994, a consensus conference (The American-European Consensus Conference on ARDS) led to the establishment of a definition for acute lung injury (ALI) and ARDS.1 ARDS is thus defined according to the following criteria: (a) relation: partial oxygen pressure/fraction of inspired oxygen (PaO2/FiO2) <200; (b) bilateral alveolar infiltrates on chest x-ray, and (c) absence of clinical signs of left heart failure or pulmonary capillary pressure <18 mm Hg. Those patients with the same criteria but a PaO2/FiO2<300 were identified as ALI (acute lung injury). This syndrome has been associated with direct damage to the lung parenchyma (pneumonia, gastric aspiration, drowning, fat or amniotic fluid embolism, trauma, inhalation of toxic fumes, and damage caused by ventilation) and with indirect damage due to an inflammatory response (sepsis, pancreatitis, shock, and transfusion, among others).2–4
In the early stages of ARDS, permeability changes leading to oedema and extravasation of inflammatory cells, which causes alterations in gas diffusion and the ventilation-perfusion ratio, which is clinically translated as hypoxaemia.3–5 The inflammatory cell response, diffuse atelectasis and oedema reduce lung distensibility, making mechanical ventilation more difficult.
Lung changes have a sudden onset following exposure to risk factors and are very persistent. The magnitude and severity of respiratory failure usually requires the start of invasive ventilatory support as a principal life support method if the treatment of the causative condition is not quick and efficient.3,6,7
The advances in ventilatory strategies have been key in improving survival. The strategy that is most consolidated and amply supported by scientific evidence is lung protective ventilation. This strategy is based on the use of a low tidal volume, around 6 ml/kg ideal body weight, which allows for a certain level of hypercapnia and guarantees pause pressures in the airway under 35 or 30 cm H2O. In spite of this optimisation in the way that mechanical ventilation is applied in these patients and other salvage treatments that have been tested,8–10 the ARDS mortality rate continues to be very high, situated around 40% in several diverse observational studies,5,11 and in a recent extensive meta-analysis.12 The causes of death for this condition are septic shock, heart failure and multi-organ failure, and brain injury.2,11
Several treatments, strategies, and adjuvant measures have been tested in recent decades, most of which have systematically failed, with no significant increase in survival (such as nitric oxide, lying in prone position, anti-inflammatory treatments, high-frequency ventilation, liquid ventilation, surfactants, and many others). The only procedure that has achieved significant advances has been the improvement in mechanical ventilation strategies. These advances, which are currently the standard treatment for mechanical ventilation in ARDS patients, are still slowly being instated into clinical practice. This strategy is known as lung protective ventilation, and is combined with the use of moderate-high PEEP. The mortality rates with these modifications in some studies are reduced by 40%–31%.10,13
Meanwhile, limiting alveolar ventilation can result in hypercapnia and acidosis that can become uncontrollable, and the severe alterations in lung mechanics can limit the lung protection strategy. These difficulties are the reason why ARDS is considered as the paradigm of difficult ventilation.
During the past few years, various methods have been developed to limit lung damage caused by ventilation by using practically “static” ventilation, allowing repair to the lungs.
The appearance of extracorporeal devices with different characteristics, such as extracorporeal membrane oxygenation (ECMO), CO2 removal devices, and other recently designed methods opens the door for even more extreme protocols for lung protective ventilation, at the same time as avoiding the associated risks and inconveniences such as extreme hypercapnia, uncontrollable acidosis, and haemodynamic alterations that are frequently present in patients with severe ARDS.
As such, this type of device allows for it to be used with a double objective: 1) perform an extreme protective ventilation with a very reduced tidal volume, decreasing the lung damage associated with MV, and 2) improve gas exchange in extreme situations in which conventional mechanical ventilation is incapable of adequately supporting this function.
ECMO and the interventional lung assist membrane (iLA) are the devices most widely developed as complementary treatment options. These extracorporeal respiratory assist and low-flow/less invasive systems in the development phase are described in detail, as well as the application of these unconventional treatments in the context of ARDS that is refractory to treatment.
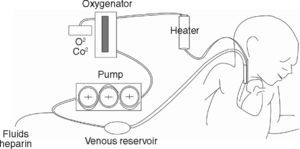
Extracorporeal Membrane Oxygenation, ECMOOxygenation by ECMO has been developed in patients that are refractory to conventional ventilatory support. ECMO is able to maintain gas exchange by using an external oxygenator that ensures oxygen supply and adequate removal of CO2 without the need for causing ventilatory damage to the respiratory system that has already been injured by the underlying disease. Depending on the patient's clinical situation and the indications for the case, ECMO can be applied using a veno-venous approach (VV) or a veno-arterial approach (VA). In both cases, blood volume is drained through an extracorporeal circuit to a centripetal pump, which then directs it to an membrane oxygenator, thus generating gas exchange with no need for participation of the pulmonary circuit. Therefore, while the patient is on ECMO, ventilatory parameters can be reduced substantially past the normal requirements for maintaining homeostasis and lung function, minimizing the damage induced by ventilation and maintaining organ function.2
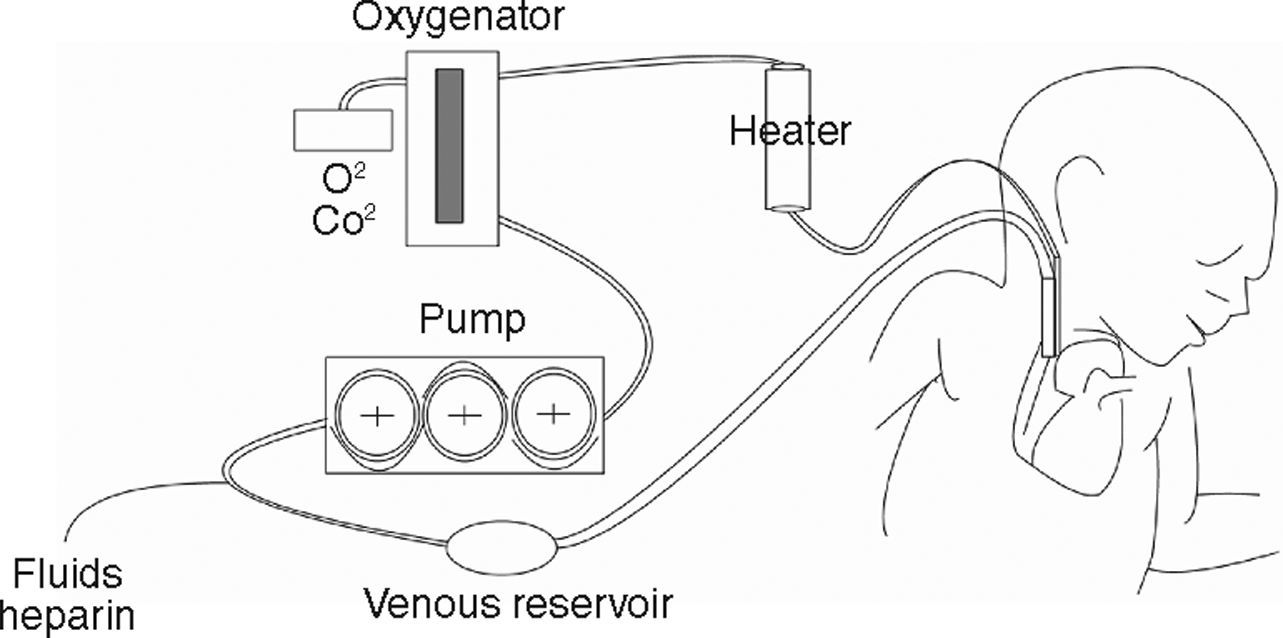
In VA ECMO, blood volume is extracted from a large-calibre vessel, normally the jugular or femoral vein, to a pump that pushes it towards the membrane oxygenator. This membrane allows for oxygenation of the haemoglobin and CO2 removal. Subsequently, blood is reintroduced into the circulatory system through a carotid or femoral arterial access after an adequate level of thermoregulation (fig. 1). In VV ECMO, both the blood volume output and re-entry accesses are placed by cannulation of the central veins. Some systems can be applied by a single double-lumen venous cannulation. VV ECMO is preferred in the majority of cases in which the objective is to provide support to lung function. Furthermore, in situations with haemodynamic instability or right/left ventricular dysfunction, VA ECMO provides both haemodynamic and lung function support, and would thus be the method of choice.2,14–16 Both VV and VA systems require complete anticoagulation in order to avoid clotting of the circuit, which constitutes a disadvantage due to the high risk of haemorrhagic complications, especially in surgery patients. An ECMO system is schematically composed of two key elements: the pump that drives blood circulation throughout the circuit and the membrane or oxygenator that allows for gas exchange. The constant technological advances in the construction and design of both elements can be key for improving the results obtained with ECMO in coming years. On the one hand, prolonged use of turbine pumps presents a problem in the destruction of blood components and haemolysis, control of the inflammatory response, and by enhancing the risk of haemorrhagic complications. Pump designs are consistently more evolved and efficient. Systems such as Levitronix Centrimag® are available, in which the traditional turbine is substituted by a rotor in which the impeller is electromagnetically levitated that does not use seals or bearings and minimises damage to the blood. The characteristics of the membrane oxygenator are just as important. These membranes imitate pulmonary capillaries by placing a fine layer between the blood and gas flows. These come with large surfaces (2–4 m2) that are then creased into multiple folds. In order to achieve a thin film of blood, a variety of geometric forms are used which use hollow fibre elements, although several different systems exist. All of these technical details can influence the performance and results of the system.
Ample experience has been acquired in the application of ECMO in paediatric and neonatal patients. The best clinical results have been achieved in this population. The paediatric experience with ECMO currently involves tens of thousands of patients with high survival rates, even surpassing 70% in experienced centres of reference.14 However, when treating adults, this type of support can be complex, demanding, and costly, with little evidence of its real efficacy and limited experience. In an international registry of patients treated with ECMO in 2005, only 1909 were adults, representing less than 5% of the total.17
Indeed, ECMO is considered as a desperate measure in adult ARDS patients. The high cost, difficulty, and complexity of monitoring such a system, as well as the need for highly prepared personnel for managing it, explain its rare use in adults. In addition, no clear evidence existed that it actually increased survival until the CESAR study.
A randomised prospective trial using ECMO in patients with ARDS that is refractory to conventional treatment reduced the initial enthusiasm surrounding this type of treatment. Zapol et al. demonstrated that no greater survival rate was observed in the group of patients treated with ECMO with respect to the group that received conventional treatment, and both groups presented a very high mortality rate (90% and 91%). This classic study was before the advent of lung protection ventilation, and so the results are difficult to extrapolate to our current situation.18 In spite of these limitations, it was possible to demonstrate an initial improvement in gas exchange in the ECMO group. Morris et al. published another randomised study comparing patients treated with ventilation and ECMO versus patients treated with only ventilation, obtaining a similar survival rate in both groups.19 However, the ECMO technology used in these studies was very different from that currently employed, and so these results, too, cannot be extrapolated to those that could be potentially obtained at the present time.
Some retrospective and prospective studies with no controls have also been carried out on the use of ECMO in refractory ARDS, which have produced better results. Lewandowski et al., in a non-randomised study, demonstrated that survival of patients treated with ECMO was significantly higher than in controls (55% versus 89%, P<.0001).20 Hemmila et al. published their experiment with 255 adult ARDS patients treated with ECMO.2 This study included patients with very severe ARDS (PAO2/FIO2<100 with FIO2 at 1.0 and alveolar-arterial gradient (A-a D02) >600 mm Hg), demonstrating a 52% survival rate for this sub-group of extremely serious patients. This retrospective study and others15,21 have been able to demonstrate an increased survival rate in highly specialised centres of reference.
The recently published CESAR study was a multi-centre randomised trial performed in the United Kingdom. This broad-scale study compares conventional ventilation methods with ECMO for the treatment of severe respiratory failure in adults.22 The study included 180 patients that were randomised into 2 groups (90 each) of controls (conventional treatment) and test cases (ECMO support). This last group presented a greater survival rate (63% versus 47%) with an improved quality of life at 6 month follow-up.23 These authors recommended transporting the ARDS patients to reference units that, in their opinion, should be rationally distributed throughout the territory in order to avoid excessive costs.
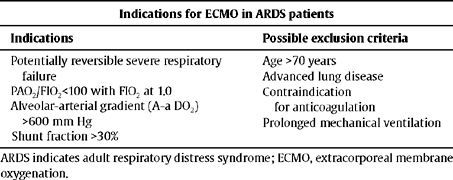
In spite of various studies that have shown the capacity for ECMO as a gas exchange support system,14,18,20,21,24–26 it is very improbable that ECMO will be extended as a treatment option in adults for various reasons:3 the lack of scientific evidence supporting the use of ECMO in adult ARDS patients, the risk implied in complete anticoagulation with heparin,14,16,18–20,25 and the elevated economic costs along with the need for highly qualified staff. Table 1 summarises the possible indications for ECMO in ARDS patients.
Proposed indications for ECMO in ARDS patients
| Indications for ECMO in ARDS patients | |
| Indications | Possible exclusion criteria |
| Potentially reversible severe respiratory failure | Age >70 years |
| Advanced lung disease | |
| PAO2/FIO2<100 with FIO2 at 1.0 | Contraindication for anticoagulation |
| Alveolar-arterial gradient (A-a DO2) >600 mm Hg | |
| Prolonged mechanical ventilation | |
| Shunt fraction >30% | |
ARDS indicates adult respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation.
In conclusion, ECMO provides improved gas exchange in severely damaged lungs, thus allowing for diminished ventilation parameters and limiting the deleterious effects of the damage induced by ventilation. However, the strongest argument against ECMO is that the increased survival rates associated with this technique have not been well established.
Interventional Lung Assist Membrane VentilatorThe most important limitation to ECMO is related to haemolysis, clotting problems associated with blood trauma, and haemolysis produced by the perfusion pump. Also, the inflammatory response and the specific technical complications for the procedure make it a high-risk and high-cost option.24,27
Kolobov and Gattioni28–31 developed a CO2 removal system using a modified form of VV ECMO (ECMO-CO2). This alternative allows for the extraction of CO2 through low-flow cannulae, but is much less efficient in terms of improving blood oxygenation. Oxygenation can be maintained by using conventional ventilation and positive pressure at the end of inspiration. With this method, the objective is to maintain oxygenation through mechanical ventilation, while the CO2 is cleared through the extracorporeal circuit. This was the reasoning for the apnoeic oxygenation which was a precursor to the interventional lung assist membrane ventilator (iLA, Novalung®).
In spite of the initially promising results,28 a randomised study comparing mechanical ventilation with ECMO and CO2 extraction showed no differences in mortality rates.19 Even so, this new concept promoted the development of more simple devices that are capable of removing CO2 and that allow for ultraprotective ventilation, avoiding some of the risks inherent to ECMO.
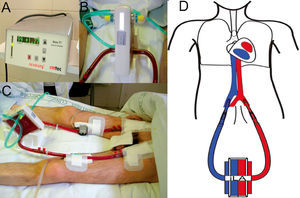
These devices (iLA) use an AV shunt with a membrane that has similar characteristics to those used in ECMO oxygenators, allowing for a highly effective CO2 removal (fig. 2). To this end, an approximate circulation of 30% of the cardiac output through the device is sufficient. This alternative reduces the complexity of conventional ECMO, by removing the venous reservoir, the centrifugal pump, and the emergency bridge, and by reducing the length of the entire circuit. In this manner, the trauma on the blood elements, haemolysis, and the coagulation problems associated with conventional ECMO are minimised. However, iLA is an inadequate treatment option for haemodynamically unstable patients or those with ventricular dysfunction, since the flow through the system is produced by the AV pressure gradient and does not use a propulsion pump with the capacity for haemodynamic support.
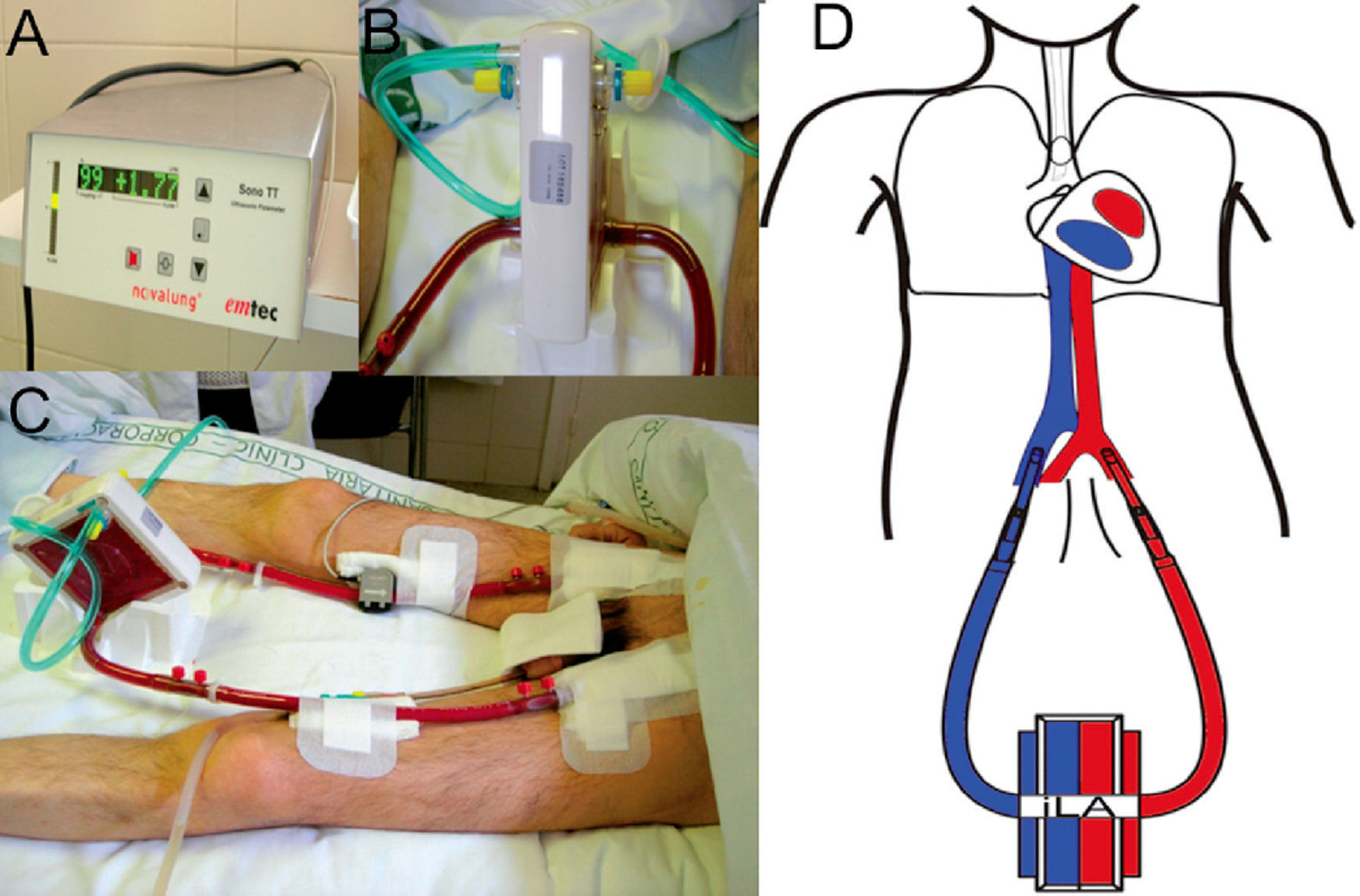
Support treatment with pumpless AV iLA in a patient with bronchiopleural fistula and severe respiratory failure. Orotracheal intubation, mechanical ventilation with PEEP at 10 cm H2O and tidal volume of 1 ml/kg ideal body weight. A) Exchange membrane and arterial and venous cannulations. B) Close up of the iLA connected to arterial and venous lines and the oxygen intake. C) Flow measure across the system, in this case 1.77 litres of blood per minute. D) AV cannulation diagram for treatment with an interventional lung assist system (iLA).
These devices were initially developed using animal models32–35 providing improved gas exchange which allowed for a reduction in airway pressure and tidal volume.36
The iLA is a low resistance device designed to function with low pulsatile flow associated with a high-diffusion exchange membrane with a layer of hydrophilic proteins and a gas exchange surface (fig. 2). The blood is propelled through this device exclusively by the peripheral blood pressure through the AV shunt by percutaneous cannulae.
Cannulation in iLA tends to be through the femoral artery outflow and the ipsi or contralateral femoral vein. Before placement, a Doppler-ultrasound exam is recommended to rule out anatomical issues, confirm the adequate calibre of the cannulae and the absence of atheroma and calcification. The calibre of the arterial cannula should be at least 20% lower than that of the artery. Other cannulation points have been tried in experimental models, such as the axillary veins, with similar results in terms of CO2 extraction.35 These experimental studies can open expectations for the development of possible implantable devices in patients with chronic hypercapnia or on the waitlist for lung transplantation, although these aspects have not yet been developed.37,38
The membrane of the device is connected to an oxygen intake. This ensures an intra-iLA pressure gradient that favours gas exchange. We must point out that this system is very inefficient at improving oxygenation, with a capacity for increasing the marginal arterial PaO2 that in the best of cases does not surpass 10%.7,39
The iLA system does ensure CO2 removal, even in practically static ventilation conditions with a minimal tidal volume. This allows for greater protection and rest for the lungs, favouring the healing of the lung damage. The tidal volume can be reduced after the placement of the iLA to levels far lower than those recommended by the ARDS Network. The CO2 removal is sufficient for obtaining normal PCO2 in arterial blood, even with tidal volumes as low as 2 ml/kg ideal body weight and even in situations of apnoeic ventilation.40,41 Several retrospective studies have demonstrated that iLA is capable of improving gas exchange in ARDS patients. Bein et al. published a study on 90 patients treated with iLA and ultraprotective ventilation for over 24 hrs, obtaining a lower mortality rate that that attributable to the severity of the patient's condition.42,43 Liebold et al. published similar results in ARDS patients with conserved haemodynamic function.27
In a recent study, Iglesias et al. published their experience using extracorporeal ventilation with iLA and ultraprotective ventilation in patients with severe respiratory failure following lung resection surgery. In this case series, the global mortality was 14%, substantially below that of an historical cohort paired by severity.40,41 In this study, a significant decrease in systemic inflammatory markers was also documented, especially interleukin-6, following the start of iLa associated with ultraprotective or apnoeic ventilation. These results suggest that iLA diminishes the inflammatory response and the lung damage caused by mechanical ventilation. The patients treated with iLA require anticoagulation with heparin, but at lower levels than with ECMO and ECMO with CO2 removal. This aspect is the most important cause for morbidity complications associated with the use of extracorporeal devices. Serial clotting controls and haemograms should be performed, since heparin-induced thrombocytopenia could be a very severe complication.44 Other complications that have been described with this technique are cannula thrombosis, iLA thrombosis, iLA or cannula leakage, ischemia of the extremities, aneurysm, and haematoma at cannulation points, which account for 15% of all observed complications. Much less frequent, under 5%, are complications related to the anticoagulation treatment, including cerebral haemorrhage, haemorrhage at the cannulation points, and to a lesser degree, shock upon connection to the system.27,42 This last complication must be planned for and compensated if necessary by using rapid fluid therapy and vasopressors.40,41
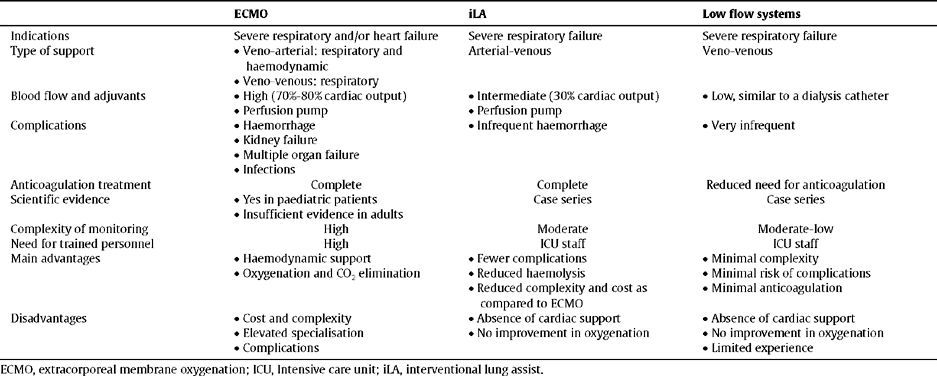
To summarise, the iLA implant is efficient, safe, and relatively simple, ensuring a high extraction of CO2 and diminishing the lung damage induced by a ventilator. The oxygenation obtained by iLA is deficient, and the indication of the device is not for treating hypoxemia, but rather to allow for a more extreme protective lung ventilation, which may therefore limit the inflammatory response associated with mechanical ventilation. Among the advantages of this device, we can include the simplification of the circuit, maintenance, and monitoring, and the much lower cost as compared to conventional ECMO. Table 2 summarises the characteristics of the different extracorporeal ventilation devises used in refractory ARDS
Characteristics of extracorporeal respiratory assist devices
| ECMO | iLA | Low flow systems | |
| Indications | Severe respiratory and/or heart failure | Severe respiratory failure | Severe respiratory failure |
| Type of support |
| Arterial-venous | Veno-venous |
| |||
| Blood flow and adjuvants |
|
|
|
|
| ||
| Complications |
|
|
|
| |||
| |||
| |||
| Anticoagulation treatment | Complete | Complete | Reduced need for anticoagulation |
| Scientific evidence |
| Case series | Case series |
| |||
| Complexity of monitoring | High | Moderate | Moderate-low |
| Need for trained personnel | High | ICU staff | ICU staff |
| Main advantages |
|
|
|
|
|
| |
|
| ||
| Disadvantages |
|
|
|
|
|
| |
|
|
ECMO, extracorporeal membrane oxygenation; ICU, Intensive care unit; iLA, interventional lung assist.
The use of iLA (Novalung®) in patients previously accepted as candidates for lung transplants, and who during their time on the waiting list suffered an acute worsening of their clinical situation, has been described in the literature.
The situation of patients on the waiting list can abruptly deteriorate, and so they can require traditional or extracorporeal ventilatory support. The potential usefulness of iLA in this context has been demonstrated in pulmonary hypertension,38,45 cystic fibrosis,46 and COPD patients.47
Particularly in COPD patients, the simplicity of this technique as compared to traditional ECMO and the reduced need for anticoagulation treatment has provided for improved prognoses, both in the state in which the patient arrives at the transplant and as a support after the transplant procedure in situations where a primary graft failure occurs.
Fisher et al38 documented their ample experience in the treatment of severe acidosis and hypercapnia in patients on the transplant waiting list with diverse subjacent diseases, including COPD, lymphangioleiomyomatosis, pulmonary fibrosis, and cystic fibrosis. In over 80% of patients, iLA successfully helped to maintain patients until the transplant, with upwards of 15 days on the device and minimal haemorrhagic complications, although membrane oxygenation replacement is frequently required when the device is used for long periods.
Low-Flow, Less Invasive VV CO2 Removal SystemsThe limited tidal volume at 6 ml/kg ideal body weight and maximum pressure at the end of inspiration at 30 cm H2O are standards for mechanical ventilation in ARDS patients. In spite of this, overdistension of alveolar units can occur in some patients even with this strategy of lung protection. On the other hand, low-volume ventilation improves survival in ARDS patients. It is possible that an ultra-protective ventilation system, with even lower tidal volumes than those described, could obtain even better survival results.
The gas exchange systems that have been evaluated in this review include a high level of instrumentation and complexity. A team of medical staff with experience in the application of these techniques is also necessary. These factors limit early application and the general use of these devices. The concept of eliminating only a part of CO2 production has been developed in order to reduce this complexity, enough to allow for a non-traumatic ventilation method with greater lung protection, avoiding acidosis. This type of approximation has initially been tested in animal models, demonstrating its safety.48 There is also a limited experiment in applying these techniques in ARDS patients.49 This type of system consists of a venous-type percutaneous vascular access using a double-lumen catheter, essentially very similar or identical to the catheters used for haemodialysis, and an membrane oxygenators such as those described in ECMO. Initially, neonatal ECMO membranes were used (DECAPSMART, decap® CO2 remover, Hemolung®) with haemofilters, although commercialised systems are currently being developed for use in adult patients. The flow through these systems is low (< 400 ml/min, less than 10% of cardiac output) and has a minimal impact on systemic haemodynamics. The quantity of blood that is maintained circulating outside of the circulatory system is low, and the anticoagulation treatment required is minimal or null, which minimises haemorrhagic complications and the need for serial clotting control. The objectives of these systems are initially more modest in terms of CO2 elimination, but at the same time increase simplicity and safety. A study with no controls evaluated 32 ARDS patients with basic ventilation and a tidal volume of 6 ml/kg ideal body weight.49 The application of this CO2 removal system diminished the tidal volume to 4 ml/kg ideal body weight, reducing the pause pressure in the airway from 29 to 25 cm H2O and maintaining PACO2 within normal limits. The study also showed a reduced systemic inflammatory response after 72 hrs of this ultraprotective ventilation, and no complications were observed associated with the procedure. This study proved the viability of the concept, although randomised trials are needed in order to confirm these results and the possible clinical benefits.
Future directionsUntil now, the support systems described have been used successfully in adults only in highly specialised centres and in select subgroups of patients. This type of ventilatory support is far from being considered as the standard treatment for ARDS patients. It is reserved as a salvage treatment in these patients and in the other situations we have described. Even so, the development of new circulatory assist pumps in the case of ECMO, the possibility of employing new, less complex systems with fewer complications, and the constant improvements in this field make new indications possible: not just in ARDS but also in severe sepsis and multiorgan failure syndrome, complicated postoperative period following thoracic surgery or transplantation, pulmonary toxicity from medications, thoracic traumas, and other situations. Potentially, applications could be developed in any situation requiring resting pulmonary status with ultraprotective ventilation, whether as a support technique or as an early preventative treatment. The possibility of more simple and portable systems with more comfortable venous vascular access also allows for speculation on the future potential of these techniques in situations with chronic hypercapnia, such as in advanced respiratory diseases, as a bridge treatment to transplantation or as a CO2 “dialysis” method.
ConclusionsThe use of extracorporeal respiratory assist systems in severe ARDS that does not respond to conventional support or in other clinical situations could contribute to improving the prognosis of select cases. The indication for these systems must be evaluated on a case-by-case basis and be considered as a salvage treatment or complementary treatment option. This type of support can provide the benefits of protection ventilation and limit lung damage associated with mechanical ventilation. Early treatment could improve the prognosis of select ARDS patients, and in complications from thoracic surgery and lung transplantation. The availability of new pumps, membranes oxygenator, and less invasive equipment could facilitate the applicability of these techniques and improve their results. Even so, the number of controlled studies is very limited, and greater scientific evidence is required in order to establish more solid recommendations and indications.