To describe the evidence- and experience-based expert consensus on the use of single-agent bronchodilators in patients with stable mild-moderate chronic obstructive pulmonary disease (COPD).
MethodsUsing Delphi methodology, a panel of 7 respiratory medicine experts was established, who, in the first nominal group meeting defined the scope, users, and document sections. The panel drew up 14 questions on the use of single-agent bronchodilators in patients with mild-moderate stable COPD to be answered with a systematic review of the literature. The results of the review were discussed in a second nominal group meeting and 17 statements were generated. Agreement/disagreement with the statements was tested among 16 different experts including respiratory medicine experts and primary care physicians. Statements were scored from 1 (total disagreement) to 10 (total agreement). Agreement was considered if at least 70% voted ≥7. The level of evidence and grade of recommendation of the systematic literature review was assessed using the Oxford Center for Evidence-based Medicine levels.
ResultsA total of 12 of the 17 statements were selected. Specific statements were generated on different profiles of patients with stable mild-moderate COPD in whom single-agent bronchodilators could be prescribed.
ConclusionsThese statements on the use of single-agent bronchodilators might improve the outcomes and prognosis of patients with stable mild-moderate COPD.
Describir el acuerdo entre expertos basado en la evidencia científica y en la experiencia sobre el uso de broncodilatadores inhalados en monoterapia en pacientes con enfermedad pulmonar obstructiva crónica (EPOC) estable leve-moderada.
MétodosSe siguió la metodología Delphi. Se seleccionó un grupo coordinador de 7 neumólogos que, en una primera reunión nominal, definieron el alcance, los usuarios, los apartados del documento y generaron 14 preguntas sobre el uso de broncodilatadores inhalados en monoterapia en pacientes con EPOC estable leve-moderada para ser contestadas por una revisión sistemática. Los resultados de la misma se discutieron en una segunda reunión nominal del grupo, en la que se generaron 17 aseveraciones. El grado de acuerdo con las aseveraciones, que se extendió a 16 expertos más (neumólogos y médicos de atención primaria), se votó según una escala de 1 (total desacuerdo) a 10 (total acuerdo), definiéndose el acuerdo como una puntuación ≥7 por al menos el 70% de los participantes. El nivel de evidencia y el grado de recomendación de la revisión sistemática se clasificaron según el modelo del Center for Evidence-Based Medicine de Oxford.
ResultadosFinalmente se aceptaron 12 de las 17 aseveraciones. Incluye aseveraciones específicas sobre distintos perfiles de pacientes con EPOC leve-moderada estable sobre los que se puede pautar un broncodilatador inhalado en monoterapia.
ConclusionesEn los pacientes con EPOC leve-moderada estable estas aseveraciones sobre el uso de la broncodilatación en monoterapia pueden ayudar en el manejo de estos pacientes.
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease among the Spanish population that places a heavy burden on both the patient and the health and social welfare system.1–3 In Spain, the EPISCAN study reported a prevalence of 10.2% among the adult population aged between 40 and 80 years,1,4,5 along with a high rate of underdiagnosis, particularly in individuals with mild disease and/or few symptoms.6
In recent years, new drugs and studies in patients with stable COPD have prompted several scientific societies and expert groups, both in Spain and in other countries, to draw up statements on the use of bronchodilators, which have been included in the consensus recently published by GOLD and other similar documents.7–14 Professionals responsible for the care of COPD patients with stable disease are currently expressing interest in the need to define and clarify the role of bronchodilators in monotherapy, in dual therapy and even as part of triple therapy in combination with inhaled corticosteroids.15
This document aims to define the degree of agreement among experts, to describe the available evidence regarding the management of patients with stable mild-to-moderate COPD, defined as FEV1≥50%, and to help clarify possible questions and areas of controversy in the use of single-agent bronchodilators. We present a series of statements that are intended to improve quality of care and assist in therapeutic decision-making, and should not be interpreted in any way as guidelines or as a COPD treatment protocol. In short, this document is presented as a tool that may optionally be adopted by clinicians involved in the management of these patients.
MethodsNominal group techniques and Delphi methodology were used to prepare this document.16 In short, this is an expert consensus document generated by a group of professionals who undertook an extensive, systematic review of the literature in order to draw up statements about topical and/or controversial aspects that may be of value to their colleagues involved in the treatment of these patients. We insist that this not a treatment protocol or guideline, but rather a clinical tool. The degree of agreement was established using Delphi methodology, and the existing level of evidence for each of the recommendations is described.
The document was prepared by distributing tasks and relaying comments to the participants, with the help of a systematic review of the literature.
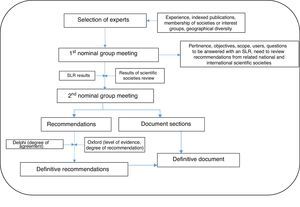
The steps followed are set out in detail below, in Fig. 1.
A group of 7 respiratory medicine experts with recognized expertise in the management of COPD patients was initially selected on the basis of the following criteria: interest, demonstrated experience in the area, Medline publications in the last 5 years, participation in research projects in the specific area of the expert statement, membership of national or international scientific societies, and geographical diversity (in order to represent different organizational healthcare models).
The objective, scope, users, and sections of the document were defined in the first nominal group meeting. Participants also agreed to perform a systematic review of the literature on different aspects of bronchodilation in COPD patients, based on a list of research questions (Table 1). These questions were generated from a series of issues considered by the experts to be topical and/or controversial in the specific area of the expert statement.
Questions Used for Designing of the Systematic Review of the Literature.
| # | Question |
|---|---|
| 1 | When and for which profile of patients with stable mild-to-moderate COPD is treatment with LABA or LAMA recommended in single-agent, dual or triple therapy? |
| 2 | What dose and regimen? |
| 3 | How effective and safe are LABAs in monotherapy compared to placebo? |
| 4 | How effective and safe are LAMAs in monotherapy compared to placebo? |
| 5 | How effective and safe are LABAs compared to LABAs in monotherapy? |
| 6 | How effective and safe are LAMAs compared to LAMAs in monotherapy? |
| 7 | How effective and safe are LABAs compared to LAMAs in monotherapy? |
| 8 | How effective and safe are LABAs/LAMAs compared to LAMA+LABA? |
| 9 | How effective and safe are LABA/ICS or LAMA/ICS compared to placebo? |
| 10 | How effective and safe are LABAs/LAMAs compared to LAMA+LABA/ICS? |
| 11 | How effective and safe are LABA+LAMA compared to LABA/ICS? |
| 12 | Are LABAs or LAMAs cost-effective in mild-to-moderate COPD? |
| 13 | What is the cardiovascular safety of LABAs and LAMAs? |
| 14 | Is any inhaler superior to another in the bronchodilator treatment of patients with mild-to-moderate COPD? |
ICS: inhaled corticosteroids; COPD: chronic obstructive pulmonary disease; LABA: long-acting β2-agonists; LAMA: long-acting antimuscarinics.
A research protocol was established and the questions generated in the previous phase (Table 1) were used to define the PICO: population (P), intervention (I), comparison (C) and outcomes (O). The PICO was used to design the search strategy and the inclusion and exclusion criteria of the review were defined in depth. Studies were selected that included adults with stable mild-to-moderate COPD (according to the different criteria developed by GOLD over time, but primarily patients with FEV1≥50% predicted value), receiving long-acting muscarinic antagonists (LAMA) (tiotropium, glycopyrronium, aclidinium, umeclidinium) and/or inhaled long-acting β2-agonists (LABA) (salmeterol, formoterol, vilanterol, indacaterol, olodaterol), irrespective of the dose, type, combinations with inhaled corticosteroids or other LABAs or LAMAs, versus a valid comparator group, placebo or other LABA or LAMA. These studies also had to include an analysis of clinical efficacy data, with at least one of the following parameters: dyspnea (functional class), exacerbation, quality of life, FEV1, lung volumes, inspiratory capacity, physical activity, exercise capacity, hospitalization, comorbidities, anxiety/depression, safety (cardiovascular, osteoporosis, mortality, etc.), or cost-effectiveness. Finally, studies with the following designs were included: meta-analyses, systematic reviews, clinical trials (>1 week duration). With regard to observational studies (including cross-sectional studies), only good quality examples were included (at least 50 patients, follow-up longer than 8 weeks, and well-defined methodology). Animal and basic science studies or other studies that did not meet any of the criteria described above were excluded.
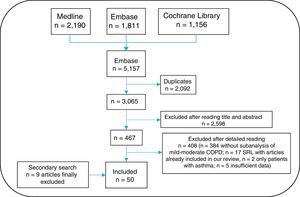
An information specialist helped generate search strategies for the different databases, using MeSH and free-text terms. Searches were limited to studies in humans, published in English or Spanish. The following bibliographic databases were consulted: Medline, Embase and Cochrane Library, between their inception and March 2016. Given the large volume of citations retrieved, the gray literature from Spanish and international congresses in the past two years was excluded from the review. A secondary manual search of the list of references of the articles finally included was then performed. All citations retrieved from the searches were entered into the EndNote system for ease of management.
Three reviewers (EL, TO, MJ) independently analyzed the articles retrieved from the search strategy. Any instances of discrepancy were resolved by a fourth person (LC). The search result was first cleaned according to the title and summary in sessions lasting up to 60 minutes. The selected articles were subsequently analyzed in detail (the article was read in full). The results of the selection of articles are shown in Fig. 2. The 3 reviewers collected the data from the studies included using specific templates designed in advance for this review. The Jadad scale was used to evaluate the methodological quality of the randomized clinical trials included, and the Oxford scale was used for the others.17
A series of provisional statements was generated from the results of the systematic review.
Phase 3. Review of the Main National and International RecommendationsThe main Spanish and international clinical practice guidelines and consensus documents were reviewed in parallel with the systematic review of the literature. These included those of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) from 2016,7 the Spanish COPD Guidelines (GesEPOC) from 2012,8 the guidelines of the National Institute for Health and Care Excellence (NICE) from 2016,9 the guidelines of the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS) the European Respiratory Society (ERS) guidelines from 2011,10 the Canadian Thoracic Society guidelines from 2008,18 and the Latin American Chest Association (ALAT) guidelines from 2011.11
Phase 4. Second Nominal Group MeetingThe results of the systematic review of the literature and provisional recommendations were presented and discussed in the second nominal group meeting, and the recommendations that would then be submitted to the Delphi process were defined to evaluate the degree of agreement.
Phase 5. Delphi SurveyA group of 23 experts in the management of patients with COPD was established that included, in addition to the panelists, other pulmonologists and primary care doctors (see acknowledgments). Experts were defined as professionals seeing >100 patients with COPD/year or who had published at least 1 article on COPD in the previous year or had presented >2 communications to conferences on COPD in the previous year. These individuals were sent an anonymous, online questionnaire containing the complete statements and instructions for rating their degree of agreement with each assertion. Degree of agreement was expressed on a Likert scale of 1 (strongly disagree) to 10 (strongly agree). Consensus was reached if the statement scored 7 or more.
Phase 6. Final Expert StatementThe results of the Delphi survey were used to draw up the definitive statement. With the assistance of the methodologist, each statement was assigned a level of evidence (LE) and a grade of recommendation (GR) according to the recommendations for evidence-based medicine from the Oxford Center for Evidence-Based Medicine,17 and an inter-expert degree of agreement (DA).
ResultsTwelve of the 17 statements generated from the Delphi survey were accepted (see results of the Delphi survey in Table 2).
Statements and Results of the Delphi Survey.
| # | Statements | Mean (SD) | Median | p25 | p75 | Min | Max | % ≥7 |
|---|---|---|---|---|---|---|---|---|
| 1 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator rather than short-acting bronchodilators is recommended | 8.44 (4.2) | 7 | 8.5 | 10 | 4 | 10 | 91% |
| 2 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with dyspnea mMRC grade ≥1 | 7.95 (4.2) | 7 | 8 | 10 | 4 | 10 | 83% |
| 3 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with CAT≥10 | 7.31 (3.5) | 6.5 | 6 | 10 | 4 | 10 | 70% |
| 4 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in symptomatic patients with cough and/or expectoration and/or wheezing. | 6.65 (5.6) | 6 | 8 | 10 | 2 | 10 | 87% |
| 5 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with a generally limited level of physical activity | 8.18 (4.2) | 7 | 8 | 10 | 4 | 10 | 87% |
| 6 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with a risk of exacerbations (≥1 severe and/or ≥2 moderate exacerbations in the previous year). | 4.24 (4.9) | 4.5 | 3 | 9.5 | 1 | 10 | 57% |
| 7 | In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with a risk of exacerbations (1 moderate exacerbation in the last year) | 7.33 (5.6) | 6 | 8 | 10 | 2 | 10 | 87% |
| 8 | In an asymptomatic patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended if FEV1 lung function values are between 50% and 65%. | 5.78 (5.6) | 5 | 7 | 10 | 1 | 10 | 83% |
| 9 | In a patient with stable mild-to-moderate COPD, the choice between 1 treatment or another will be assessed on the basis of other aspects, such as self-management skills, patient preferences, and treatment adherence | 8.41 (2.8) | 8 | 7.5 | 10 | 6 | 10 | 87% |
| 10 | In a patient with stable mild-to-moderate COPD, the choice between 1 treatment or another will also be assessed on the basis of other aspects, such as comorbidities, patient age, drug safety profile | 7.89 (4.9) | 6.5 | 8.5 | 10 | 3 | 10 | 91% |
| 11 | Current evidence is insufficient to justify the prescription of any specific bronchodilator (LABA/LAMA) as initial treatment (single-agent or dual bronchodilation) in patients with stable mild-to-moderate COPD | 4.95 (4.9) | 5.5 | 5 | 9 | 2 | 10 | 61% |
| 12 | Starting dual bronchodilation is recommended in patients with mild-to-moderate COPD refractory to monotherapy (LE 1b; DR A; DA 91%). | 8.50 (4.9) | 6.5 | 9 | 10 | 3 | 10 | 91% |
ACOS: asthma-chronic obstructive pulmonary disease overlap syndrome; CAT: chronic obstructive lung disease assessment test; ICS: inhaled corticosteroids; SD: standard deviation; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; LABA: long-acting β2-agonists; LAMA: long-acting antimuscarinics; Max: maximum; Min: minimum; mMRC: modified Medical Research Council; p25: 25th percentile; p75: 75th percentile.
The first action, considered fundamental, is to perform a comprehensive study of the COPD patient to confirm diagnosis and evaluate the disease.
The therapeutic objective is clinical improvement of cough, expectoration, dyspnea, wheezing, impact on physical activity and quality of life. It is important that symptoms are appropriately assessed, and other causes are ruled out or excluded.
The following statements must be adapted according to the clinical criteria observed in each individual patient, taking into consideration age, cognitive level, skill in the use of devices, and presence of comorbidities and comorbidities. Adherence to other complementary therapies such as smoking cessation, vaccination, optimization of body weight and pulmonary rehabilitation must also be considered.
Moreover, in this respect, while mild-to-moderate COPD is taken as FEV1≥50%, it is equally important that patients are assessed individually according to their specific FEV1 value, since this range includes a broad spectrum of severity: values close to 50% (even up to 65%) are not the same as values greater than 80%.
Starting Treatment With Long-acting Single-agent BronchodilatorsThe panel believes that there are a number of situations in which patients with stable mild-to-moderate COPD are candidates for treatment with long-acting single-agent bronchodilators.
Statement 1“In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator rather than short-acting bronchodilators is recommended” (LE 2a; DR B; DA 91%).
Evidence has shown that in this type of patient, a long-acting bronchodilator is more effective than a short-acting bronchodilator.19,20
Statement 2“In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with dyspnea mMRC grade≥1” (LE 1b; DR A; DA 83%).
Several studies have demonstrated their efficacy in patients with dyspnea.21 This symptom correlates with other variables that determine health status and is a predictor of mortality.22,23
Statement 3“In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with CAT≥10” (LE 2a; DR B; DA 70%).
The COPD Assessment Test (CAT) is a tool which evaluates the impact of symptoms and quality of life in COPD patients.24 It has been used in various studies on the efficacy of long-acting bronchodilators.25
Statement 4“In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in symptomatic patients with cough and/or expectoration and/or wheezing” (LE 1b; DR A; DA 87%).
Several published studies have evaluated the effect of long-acting bronchodilator on symptoms related to airway involvement of these patients, and satisfactory results have been obtained.26–29
Statement 5“In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with a generally limited level of physical activity” (LE 1b; DR A; DA 87%).
Physical activity is correlated with an improved prognosis and quality of life in patients with COPD. Bronchodilation has been observed to have a favorable effect on this parameter among this patient population.28,30–34
Statement 6The level of agreement achieved for the following statement was insufficient: “In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with a risk of exacerbations (≥1 severe and/or ≥2 moderate exacerbations in the last year)”.
Although most experts support this statement, the required level of agreement was not achieved. There may be several reasons for this lack of agreement: scientific evidence emerging in recent years continues to support the use of LAMA/LABA/inhaled corticosteroids in patients with an exacerbator profile, although the publication of the FLAME study in a large patient cohort has shown that dual bronchodilation is superior to the combination of salmeterol/fluticasone in the prevention of exacerbations.14 Nevertheless, most patients in this series had severe or very severe COPD. Moreover, the definition and the type of exacerbation influence the choice of treatment.
Statement 7“In a patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended in patients with a risk of exacerbations (1 moderate exacerbation in the previous year)” (LE 1a; DR A; DA 87%).
Recent studies in monotherapy with dual bronchodilation support this statement.14,35–37
Statement 8“In an asymptomatic patient with stable mild-to-moderate COPD, starting inhaled treatment with a long-acting bronchodilator is recommended if FEV1 lung function values are between 50% and 65%” (LE 5; DR D; DA 83%).
Although a percentage of patients with this degree of pulmonary involvement might be asymptomatic or minimally symptomatic, the initial choice of a bronchodilator therapy will have prognostic implications in the medium to long term.38
Statement 9“In a patient with stable mild-to-moderate COPD, the choice between 1 treatment or another will be made on the basis of other aspects, such as self-management skills, patient preferences, and treatment adherence” (LE 5; DR D; DA 87%).
Treatment should be individually selected with the aim of achieving greater adherence, and a number of criteria that facilitate correct use will be taken into account: good health education, the patient's clinical and functional profile, and personal preferences.39
Statement 10“In a patient with stable mild-to-moderate COPD, the choice between 1 treatment or another will also be assessed on the basis of other aspects, such as comorbidities, patient age, drug safety profile” (LE 5; DR D; DA 91%).
COPD is a disease that is associated with different comorbidities affecting the patient's prognosis. Patients with many of these comorbidities have been excluded from clinical trials designed to evaluate pharmacological efficacy. However, the available evidence shows that bronchodilators are safe drugs, and that there is no evidence that single-agent LABA or LAMA increase cardiovascular risk in the short-to-medium-term in patients with mild-to-moderate COPD.36,40,41
Statement 11An insufficient level of agreement was reached for the following statement: “Current evidence is insufficient to justify the prescription of any specific bronchodilator (LABA/LAMA) as an initial treatment (single-agent or dual bronchodilation) in patients with stable mild-to-moderate COPD”.
Available scientific evidence to date is insufficient to determine the selection of 1 bronchodilator or another, due to the lack of studies directly comparing these treatments in specifically comparable populations. Perhaps the “class effect” must be taken into account when selecting 1 group or another.
Statement 12“Starting dual bronchodilation is recommended in patients with mild-to-moderate COPD refractory to monotherapy” (LE 1b; DR A; DA 91%).
We use “refractory” to define patients who remain symptomatic and/or have exacerbations despite single-agent treatment with LAMA or LABA.14
The following approach is recommended in these patients: (1) assess treatment adherence (and reasons for discontinuation in case of poor adherence, safety problems) and intercurrent situations that may reduce therapeutic efficacy (smoking); (2) reconsider the diagnosis: rule out asthma-COPD overlap (ACO), and (3) adequately assess comorbidities.
The panel recommends evaluating the response after 3 months, with a view to adjusting treatment (scaling up or down).
DiscussionFollowing the recent incorporation of new drugs in the therapeutic arsenal for the treatment of COPD and the publication of various research studies, the diagnostic and therapeutic management of patients with stable COPD has become the subject of numerous national and international guidelines and recommendations.7,14,42 However, treatment indications are less well defined and more controversial for certain individuals among the mild-to-moderate COPD subgroup.
If clinical practice is to improve, explicit statements addressing important aspects such as the evaluation of these patients and the appropriate use of inhaled bronchodilators must be available. Although the evidence is scant in some areas and there may be some limitations to implementing the recommendations in clinical practice, this document presents a series of statements that in our opinion are relevant and useful for clinicians.
COPD is a heterogeneous disease that is currently generating some controversy in terms of patient classification. Conventionally, spirometric classification of lung function was used as a criterion for severity, although it is not clear whether the prognostic implications of spirometry are as important as the symptomatic impact of dyspnea, loss of quality of life, and exacerbations. The scientific evidence on the implications of early treatment of COPD is currently being widely discussed, and there is no doubt of the pivotal importance of bronchodilation in the pharmacological treatment of these patients. The progress of the mild-to-moderate COPD patient will depend on appropriate diagnostic management and the administration of treatment that will modify the natural history of the disease, or in other words, the prognosis of these patients.
Despite innovative strategies involving dual or triple bronchodilator therapy, there is now sufficient quality of evidence to underline the importance of the use of single-agent inhaled bronchodilation in the initial treatment of stable COPD patients classified as mild or moderate with different characteristics: dyspnea mMRC ≥1 (level of evidence 1b), presence of bronchial symptoms (cough and/or expectoration and/or wheezing) (level of evidence 1b), limitation in physical activity (level of evidence 1b).35,42
This document shows that Spanish experts agree on a considerable number of statements regarding the use of inhaled bronchodilators in monotherapy for stable COPD.
Interestingly, however, no agreement was reached on the indication of this treatment in exacerbators or in patients with a risk of exacerbation, suggesting that this remains a controversial topic, from both a diagnostic and a therapeutic point of view, despite recent studies that will clearly mark a turning point in recommendations and guidelines. Another area of uncertainty generated by the current lack of valid evidence is highlighted by the lack of agreement on the choice of a particular bronchodilator. In daily practice, however, there is a slight trend toward using LAMA as an initial therapy in a patient with a risk of exacerbation.
Incidentally, other aspects such as bronchodilation were discussed and evaluated while preparing the document, although not in as much depth as monotherapy (bronchodilation was evaluated in the systematic review). The expert group considered, for example, that treatment could be initiated with dual bronchodilation (LABA+LAMA) in patients with stable mild-to-moderate COPD and dyspnea mMRC≥2.
Studies have been published in which dual bronchodilation (LAMA+LAMA), in the short term at least (less than 6 months), was seen to be superior to monotherapy in improving functional parameters such as FEV1, but no clear difference was observed between dual and single-agent therapy in terms of lung volumes, inspiratory capacity, dyspnea, and/or exacerbations in these patients.35,42–47 No differences were found in the safety profile. Patients previously treated with LAMA have a worse response to dual bronchodilation than those who received it de novo.44
Dual therapy in patients with ≥1 severe and/or ≥2 moderate exacerbations in the previous year was also assessed. The recent publication of the FLAME study supports this approach in a certain patient population (severe or very severe COPD).14
Patients with ACO, on the other hand, can start treatment with LABA+inhaled corticosteroids, since these patients present a higher degree of bronchial eosinophilic inflammation that would explain their greater clinical and spirometric response to inhaled corticosteroids.12,13,37
It was also suggested that withdrawal of inhaled corticosteroids can be considered in patients with stable mild-to-moderate COPD without ACO. These drugs are overused, and the recommendation for their withdrawal is justified by their well-documented high rate of adverse effects. Some recent publications also suggest that patients with stable mild-to-moderate COPD and no exacerbation in the previous year and appropriate bronchodilation, may also be good candidates for the discontinuation of inhaled corticosteroids.48
Finally, we did not believe it appropriate to assess triple therapy as initial treatment in patients with stable mild-to-moderate COPD.
The objective of these statements is to help dispel the doubts or uncertainties that professionals involved in the treatment of mild-to-moderate COPD may encounter in their routine clinical practice. They are, however, merely expert opinions and lack in the scientific study-based evidence needed to support the more controversial issues. As such, they are subject to the bias or limitations inherent in this type of document.
Another of the limitations of these statements is rooted in the lack of studies and insufficient scientific evidence supporting the use of monotherapy in certain situations that could be termed special: should we approach men and women differently? Should active smokers or former smokers be managed differently? How should stage 0 (at risk) COPD be managed? Should smokers or former smokers with mild asymptomatic COPD be treated? Some recent studies have suggested that future lines of research should head in this direction.
Finally, as in any chronic disease, patients may perceive their symptoms differently, irrespective of their degree of severity, and this may influence their response and adherence to treatment. Health education will be the key to selecting the best treatment, although further studies are needed to standardize the method used to evaluate response to prescribed inhaled treatment.
ConclusionsThis document has generated a series of statements agreed upon by Spanish experts on the use of inhaled bronchodilator therapy in monotherapy in patients with stable mild-to-moderate COPD. Consensus was reached regarding the indication of monotherapy as an initial treatment in patients with dyspnea mMRC≥1, cough-expectoration-wheezing, CAT≥10, limitation in physical activity. The use of dual bronchodilation is recommended as an initial treatment in patients with mild-to-moderate COPD with dyspnea mMRC≥2 or in patients refractory to monotherapy. The lack of agreement on the treatment of patients with a history of exacerbations and the choice of a specific type of bronchodilator highlights the uncertainty surrounding these issues and the pressing need for future studies in these areas.
FundingThe project was funded by Gebro Pharma, which did not intervene in the design of the project or in the development of the recommendations.
Conflict of InterestsJuan Antonio Riesco Miranda states that he has received fees for lectures and/or scientific consultancy from AstraZeneca, Boehringer-Ingelheim, Chiesi, Gebro Pharma, Group Ferrer, GSK, Laboratories Esteve, Menarini, Novartis and Pfizer.
Inmaculada Alfageme Michavila states that in the last three years she has received fees for lectures and/or scientific consultancy from AstraZeneca, Gebro Pharma, Roche, GSK, Laboratorios Esteve, Novartis, Teva and Chiesi.
Bernadino Alcázar Navarrete states that he has received fees for lectures and/or scientific consultancy from AstraZeneca, Boehringer-Ingelheim, Chiesi, Gebro Pharma, Grupo Ferrer, GSK, Laboratorios Esteve, Laboratorios Menarini, Novartis, and Rovi.
Ciro Casanova Macario states that in the last three years he has received fees for lectures and/or scientific consultancy from AstraZeneca, Boehringer-Ingelheim, Gebro Pharma, GSK, Laboratories Esteve, Menarini, Novartis, and Rovi.
Bartolome Celli states that he received expenses from AstraZeneca for the division where he works to conduct COPD studies. He has received fees for consultancy from Glaxo Smith Kline, Boehringer-Ingelheim, AstraZeneca, Medimmune, and Novartis. Neither he nor his family have economic interests in any company, including any related to tobacco.
Juan P. de-Torres states that he received fees for lectures and/or scientific consultancy from AstraZeneca, Chiesi, Gebro Pharma, GSK, Menarini, Novartis, and Teva.
Carlos A. Jiménez-Ruiz states that he has received fees for speaking engagements and/or scientific consultancy from AstraZeneca, Boehringer-Ingelheim, Gebro Pharma, Laboratorios Esteve, Menarini, Novartis and Pfizer.
Our thanks to all the professionals who participated in the Delphi survey: Doctors José Luis López-Campos, Francisco Javier Callejas, Carlos Cabrera López, Elsa Naval Sendra, Patricia Sobradillo, Juan Luis García Rivero, Juan José Soler Cataluña, Marc Miravitlles Fernández, Juan Antonio Trigueros, Agustín Valido Morales, José María Marín Trigo, José Antonio Quintano, Jesús Molina París, Eduardo Márquez, Myriam Calle Rubio, Adolfo Baloira Villar.
Please cite this article as: Riesco Miranda JA, Alcázar B, Alfageme I, Casanova C, Celli B, de-Torres JP, et al. Documento de expertos del uso de broncodilatadores inhalados en monoterapia en el tratamiento de la EPOC estable leve-moderada. Arch Bronconeumol. 2017;53:574–582.