Bronchiolitis Obliterans Syndrome (BOS) is a debilitating disease with limited treatment options that threatens both the quality of life and long-term survival of lung transplant (LTx) recipients. This retrospective longitudinal case–control study was performed to compare the long-term functional evolution of LTx recipients with and without BOS.
MethodsTwenty-four LTx recipients with BOS (BOS=Cases) and 24 without BOS (NON-BOS=Controls) were selected and individually matched according to age, gender, diagnosis and LTx characteristics. Measurements of 6-minute walking distance (6MWD), symptoms of dyspnea (BORG CR-10 scale), and comprehensive pulmonary function testing were performed before LTx and at annual follow-up assessments after LTx.
ResultsPeak FEV1 after LTx was similar in both groups [FEV1 (% predicted) 101±25 vs. 101±31, p=0.96] and BOS diagnosis in cases was established 3.6±2.5 years after LTx. At the final follow-up assessment (6.5±3.2 years after LTx) FEV1 (% predicted) was 86±34 in NON-BOS vs. 44±17 in BOS (p<0.001). Evolution of 6MWD was different between groups (group by time interaction: p=0.002). Borg dyspnea scores were also significantly different between groups at the final evaluation (NON-BOS 3.3±1.7 vs. BOS 5.0±2.2; p=0.024).
ConclusionsWe observed gradual reductions in functional exercise capacity and increasing symptoms of dyspnea in patients who developed BOS after LTx. As such, prospective studies seem warranted to explore whether rehabilitative interventions might be useful to improve symptoms and slow down deterioration of exercise capacity in these patients from the onset of BOS.
El síndrome de bronquiolitis obliterante (BOS) es una enfermedad debilitante con opciones de tratamiento limitadas que amenaza tanto la calidad de vida como la supervivencia a largo plazo de los trasplantados pulmonares (LTx). Este estudio longitudinal retrospectivo de casos-controles se realizó para comparar la evolución functional de los LTx con y sin BOS a largo plazo.
MétodosSe seleccionaron 24 LTx con BOS (BOS=casos) y 24 sin BOS (NON-BOS=controles). Los casos y controles se emparejaron individualmente atendiendo a la edad, el sexo, el diagnóstico y las características del trasplante. Las mediciones incluyeron: la distancia recorrida en la prueba de 6min marcha (6MWD), la disnea (BORG CR-10) y la función pulmonar, antes del LTx y anualmente en el post-LTx.
ResultadosEl FEV1 pico post-LTx fue similar en ambos grupos (FEV1 [% predicho] 101±25 vs. 101±31; p=0,96) y el diagnóstico de BOS en los casos se estableció a los 3,6±2,5 años del LTx. En la evaluación del seguimiento final (6,5±3,2 años tras el LTx) el FEV1 (% predicho) fue del 86±34 en los NON-BOS vs. 44±17 en los BOS (p<0,001). La evolución del 6MWD fue diferente entre grupos (interacción tiempo por grupo: p=0,002). La puntuación de disnea en la evaluación final también fue diferente significativamente entre grupos (NON-BOS 3,3±1,7 vs. BOS 5,0±2,2; p=0,024).
ConclusionesSe ha observado una reducción gradual de la capacidad funcional de ejercicio y un incremento de la disnea en los pacientes con BOS tras el LTx. Así pues, parecen pertinentes estudios prospectivos para examinar si la rehabilitación puede mejorar la sintomatología y enlentecer el deterioro de la capacidad de ejercicio en los pacientes que desarrollan el BOS tras el trasplante.
Bronchiolitis Obliterans Syndrome (BOS) is a debilitating disease with limited treatment options that threatens both the quality of life and long-term survival of lung transplantation (LTx) recipients.1 The syndrome has been associated with restrictions in physical mobility and decreased energy.2 According to data from the registry of the International Society for Heart and Lung Transplantation (ISHLT) BOS affects at least 50% of lung recipients who survive beyond 5 years and is the leading cause of death for recipients who survive beyond 1 year post-transplant.3 Expected survival before and after transplantation has been shown to depend on prognostic variables of candidates for LTx such as functional exercise (walking) capacity.4,5 Only two studies6,7 have so far reported on the evolution of functional exercise capacity (FEC) in long-term LTx survivors. Gerbase and colleagues’ study is the only one to date that compared long-term evolution of FEC (6-minute walking distance) after LTx between BOS and NON-BOS patients.7 They observed that the distance covered during the 6-minute walking test (6MWD) remained fairly stable over time in patients who developed BOS.7 Based on these observations they concluded that the development of moderate to severe BOS did not seem to prevent lung recipients from walking independently and pursuing an autonomous life.7 In contrast with these findings Rutherford and colleagues observed in 10-year LTx survivors (including a significant fraction of patients with BOS; 46% with BOS Grade 1 and 36% with BOS Grade ≥2) significant reductions in the physical functioning domain of the SF-36 in comparison with both normative and chronic illness data.6 Based on these conflicting data and after contrasting clinical impressions in our own center we decided to formally study the evolution of FEC in LTx survivors with BOS diagnosis. In order to avoid the influence of possible confounding factors (age, gender, pre-LTx diagnosis, year of LTx and LTx characteristics) we individually matched BOS and non-BOS patients in a retrospective longitudinal case–control study design. Furthermore, only data from patients with complete follow-up were considered for analysis to avoid survival bias. We aimed to compare the evolution of functional exercise capacity, as well as symptoms of dyspnea and pulmonary function in surviving LTx recipients with and without BOS diagnosis.
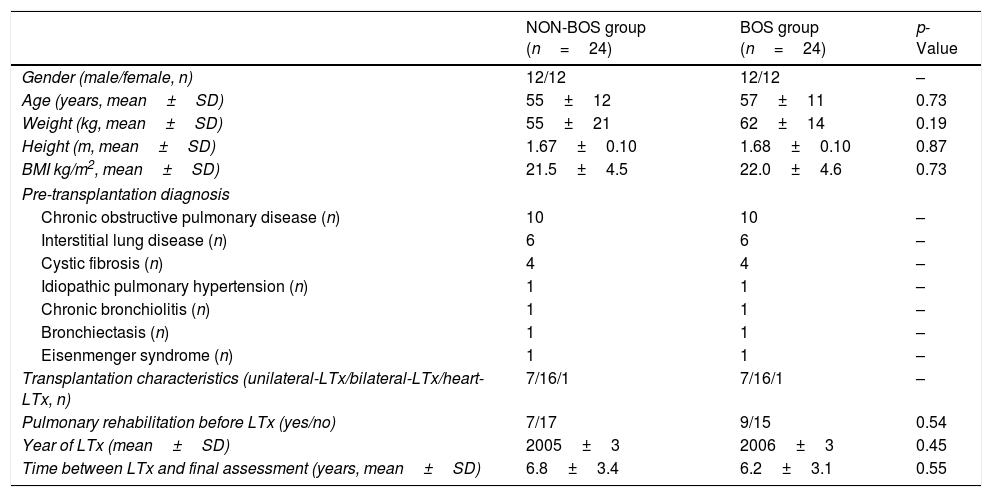
MethodsSetting, participants and study designPatients who had undergone LTx in the University Hospital Leuven after June 1999, subsequently developed BOS, and who were still alive in August 2012 were retrospectively reviewed from the day of their transplantation until August 2012. The study was approved by the Hospital's Ethics Committee and all patients had provided written informed consent to access their data from the LTx database. Twenty-four surviving LTx recipients with BOS diagnosis Grade ≥2 (BOS=Cases) were identified according to criteria established by the ISHLT.8 The reason to exclude BOS Grade 1 from the study was to ensure a better correlation of symptoms with BOS. For each BOS case we individually selected an LTx recipient without BOS diagnosis (NON-BOS=Controls). The controls were individually matched to the cases according to age, gender, pre-transplant diagnosis, year of LTx, and LTx characteristics (Table 1). Additionally, the age and date of the transplantation took into account the closest to the matched case patient (BOS). In the 75% BOS patients (n=18), the difference between days were ≤1 year. The main reason to include NON-BOS patients with more than 1 year difference with respect to the date of LTx in comparison to BOS patients was the prevalence of interstitial lung disease without BOS.
General characteristics of LTx recipients with and without Bronchiolitis Obliterans Syndrome (BOS).
| NON-BOS group (n=24) | BOS group (n=24) | p-Value | |
|---|---|---|---|
| Gender (male/female, n) | 12/12 | 12/12 | – |
| Age (years, mean±SD) | 55±12 | 57±11 | 0.73 |
| Weight (kg, mean±SD) | 55±21 | 62±14 | 0.19 |
| Height (m, mean±SD) | 1.67±0.10 | 1.68±0.10 | 0.87 |
| BMI kg/m2, mean±SD) | 21.5±4.5 | 22.0±4.6 | 0.73 |
| Pre-transplantation diagnosis | |||
| Chronic obstructive pulmonary disease (n) | 10 | 10 | – |
| Interstitial lung disease (n) | 6 | 6 | – |
| Cystic fibrosis (n) | 4 | 4 | – |
| Idiopathic pulmonary hypertension (n) | 1 | 1 | – |
| Chronic bronchiolitis (n) | 1 | 1 | – |
| Bronchiectasis (n) | 1 | 1 | – |
| Eisenmenger syndrome (n) | 1 | 1 | – |
| Transplantation characteristics (unilateral-LTx/bilateral-LTx/heart-LTx, n) | 7/16/1 | 7/16/1 | – |
| Pulmonary rehabilitation before LTx (yes/no) | 7/17 | 9/15 | 0.54 |
| Year of LTx (mean±SD) | 2005±3 | 2006±3 | 0.45 |
| Time between LTx and final assessment (years, mean±SD) | 6.8±3.4 | 6.2±3.1 | 0.55 |
Abbreviation: LTx, lung transplantation.
Main outcomes were functional exercise capacity (6MWD), Borg CR-10 dyspnea and leg fatigue scores9 administered at the end of the walking test, and pulmonary function variables. All data were collected in every patient before LTx (while on the waiting list), immediately following hospital discharge after LTx, and on an annual basis following LTx. For analysis, we selected the values recorded at four time-points: (1) before LTx (pre-LTx); (2) immediately after hospital discharge following LTx (post-LTx); (3) at the follow-up visit when the highest forced expiratory volume in one second was measured (peak FEV1); and (4) at the most recent visit (recent value). Functional exercise performance was measured by a 6MWD test in a 50-meter corridor.10 The better of two tests was used and expressed as a percentage of predicted normal values established in our laboratory.10 Borg CR-10 symptom scores for dyspnea and leg fatigue were administered at the beginning and at the end of the test. Pulse oximetry (SpO2) was assessed continuously throughout the test (Model 8500 Handheld Pulse Oximeter, Nonin Medical B.V., Amsterdam, NL) and values were recorded at rest and immediately after completion of the test. Comprehensive pulmonary function testing was performed according to international guidelines.11–13 Values are expressed as a percentage of the predicted normal values for European Caucasians.14
Statistical analysisStatistical analysis were performed with SPSS for Windows (Release15.0.1, SPSS Inc., Chicago, IL, USA, 2006) and SAS for Windows (Version 9.3, SAS Institute Inc., Cary, NC, USA, 2011). Data are presented as mean±standard deviation for quantitative variables and percentages for categorical variables throughout the manuscript unless indicated otherwise. Normality as well as other statistical assumptions were tested before using data-modeling techniques. Baseline characteristics were compared between LTx recipients with and without BOS by unpaired t-tests and Chi-square tests. Repeated measures analysis to compare changes in evolution of functional outcomes after LTx between BOS and NON-BOS patients were performed in SAS. Outcomes between groups (BOS vs. NON-BOS) were compared with a mixed model analysis (Proc Mixed). “Time” and “Group” and interactions between these variables were considered as fixed effects. Since all measurements were clustered within a patient, the latter was considered as a random effect for between group comparisons. Differences between the BOS and the NON-BOS at the most recent assessment were compared with a general linear model (GLM Repeated Measures procedure, SPSS for Windows). Results at the most recent assessment were entered into the model as ‘within subject factors’. Group (BOS or NON-BOS) was entered as a ‘between subject factor’ and assessments performed before the transplantation (baseline) were entered as covariates. Pearson correlation analysis were performed in SPSS to examine the linear association between changes in symptoms and changes in functional exercise capacity (Δ=differences between ‘Peak FEV1’ and ‘Recent value’ assessments). An alpha of less than 0.05 was considered as the threshold for statistical significance.
ResultsAll 24 LTx recipients (50% females; 57±11 years) diagnosed with BOS Grade ≥2 who were transplanted after June 1999 and who were still alive in August 2012 were selected for participation in the study (5.9% of LTx survivors in our center). Patients from the NON-BOS group were individually matched according to age, gender, pre-transplant diagnosis, year of transplantation and LTx characteristics which resulted in comparable baseline characteristics with regard to these variables (Table 1). Groups were also comparable regarding height, pulmonary function, 6MWD and symptoms at the pre-LTx measurement (Tables 1 and 2). The most common pre-transplant diagnosis was chronic obstructive pulmonary disease (42%), followed by interstitial lung disease (25%) and cystic fibrosis (17%). Approximately two-third of the performed surgical procedures were bilateral-LTx (Table 1). We assessed also other variables of interest to control for potential confounding: a similar proportion of patients from both groups performed a pre-transplantation rehabilitation program (29% vs. 38%, p=0.540; Table 1) and around 40% of candidates in both groups needed oxygen supplementation during the 6MWD pre-LTx (2.1±0.9 l vs. 2.6±0.2 l, p=0.432) in the NON-BOS and BOS group, respectively. Results of measurements performed at four time-points: (1) pre-LTx; (2) post-LTx; (3) peak FEV1; and (4) recent value, are summarized and illustrated in Table 2 and Fig. 1. Measurements pre-LTx were performed 170±192 days and 123±111 days before transplantation in the NON-BOS and BOS group, respectively (p>0.05). The peak FEV1 value was achieved 1026±736 days (2.8±2.0 years) and 479±384 days (1.3±1.1 years) after transplantation in the NON-BOS and BOS group, respectively (p=0.002). In the cases, BOS diagnosis was established on average 1305±932 days (3.6±2.6 years) after LTx. Final measurements were performed 2466±1242 days after LTx in the BOS group and after 2258±1128 days in the NON-BOS group (6.5±3.2 years after LTx on average for both groups; p>0.05).
Pulmonary function, walking capacity and symptoms for each group at the four measured time points: values are expressed as mean±SD.
| Pre-LTx | Post-LTx | Peak FEV1 | Recent Value | Interaction effect | GLM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NON-BOS (n=24) | BOS (n=24) | p-Valuea | NON-BOS (n=24) | BOS (n=24) | p-Valuea | NON-BOS (n=24) | BOS (n=24) | p-Valuea | NON-BOS (n=24) | BOS (n=24) | p-Valuea | p-Valueb | p-Valuec | |
| FEV1 (l) | 0.99±0.43 | 1.00±0.56 | 0.944 | 2.04±0.98 | 2.14±0.87 | 0.715 | 3.01±1.25 | 3.06±1.36 | 0.881 | 2.52±1.24 | 1.23±0.53 | <0.001 | <0.001 | <0.001 |
| FEV1 (% predicted) | 34±17 | 35±23 | 0.833 | 67±25 | 70±18 | 0.649 | 101±31 | 101±25 | 0.964 | 86±34 | 44±17 | <0.001 | <0.001 | <0.001 |
| FVC (l) | 2.02±0.91 | 2.23±1.22 | 0.516 | 2.28±1.01 | 2.48±0.86 | 0.476 | 3.64±1.41 | 3.64±1.31 | 0.992 | 3.31±1.52 | 2.43±0.70 | 0.015 | 0.001 | 0.013 |
| FVC (%predicted) | 55±23 | 60±29 | 0.533 | 62±20 | 68±17 | 0.257 | 102±29 | 101±19 | 0.798 | 93±31 | 73±20 | 0.012 | 0.005 | 0.011 |
| FEV1/FVC (%) | 55±24 | 52±26 | 0.708 | 89±12 | 86±11 | 0.293 | 82±10 | 83±11 | 0.957 | 75±14 | 51±16 | <0.001 | 0.002 | <0.001 |
| FRC (%predicted) | 136±75 | 148±77 | 0.646 | 128±34 | 96±35 | 0.072 | 96±21 | 99±33 | 0.769 | 92±28 | 115±34 | 0.023 | 0.707 | 0.140 |
| TLC (%predicted) | 95±35 | 97±38 | 0.897 | 87±17 | 78±20 | 0.341 | 87±17 | 88±20 | 0.894 | 87±20 | 91±18 | 0.596 | 0.382 | 0.786 |
| TLCO (%predicted) | 31±8 | 38±22 | 0.279 | 51±25 | 47±16 | 0.689 | 57±17 | 56±16 | 0.851 | 56±17 | 53±13 | 0.546 | 0.245 | 0.974 |
| 6MWD (m) | 313±149 | 323±122 | 0.830 | 289±128 | 327±154 | 0.059 | 537±107 | 521±157 | 0.680 | 514±158 | 447±167 | 0.186 | 0.002 | 0.053 |
| 6MWD (%predicted) | 43±22 | 48±22 | 0.560 | 39±18 | 48±21 | 0.168 | 76±15 | 74±20 | 0.651 | 74±20 | 64±23 | 0.133 | 0.029 | 0.039 |
| Borg dyspnea | 6.2±2.9 | 4.7±3.0 | 0.148 | 3.8±2.8 | 2.4±1.6 | 0.052 | 2.1±1.7 | 1.8±1.5 | 0.543 | 3.3±1.7 | 5.0±2.2 | 0.011 | 0.024 | 0.123 |
| Borg leg fatigue | 4±3 | 3±3 | 0.437 | 6±2 | 5±2 | 0.152 | 4±3 | 5±3 | 0.256 | 5±3 | 4±3 | 0.589 | 0.310 | 0.370 |
| SpO2 bf 6 MWT (%) | 92±5 | 90±9 | 0.315 | 97±2 | 97±2 | 0.978 | 97±3 | 97±2 | 0.916 | 98±2 | 96±2 | 0.001 | 0.256 | 0.173 |
| SpO2 af 6 MWT (%) | 81±9 | 79±21 | 0.737 | 92±6 | 94±4 | 0.308 | 94±3 | 94±3 | 0.887 | 95±3 | 90±4 | 0.001 | 0.253 | 0.448 |
Abbreviations: LTx, lung transplantation; BMI, body mass index; FEV1, forced expiratory volume in 1Ys; FVC, force vital capacity; FRC, functional residual capacity; TLC, total lung capacity; TLCO, transfer factor of the lung for carbon monoxide; 6MWD, 6-min walking distance; Borg Scale (0–10); SpO2, saturation oxygen; bf, before; af, after.
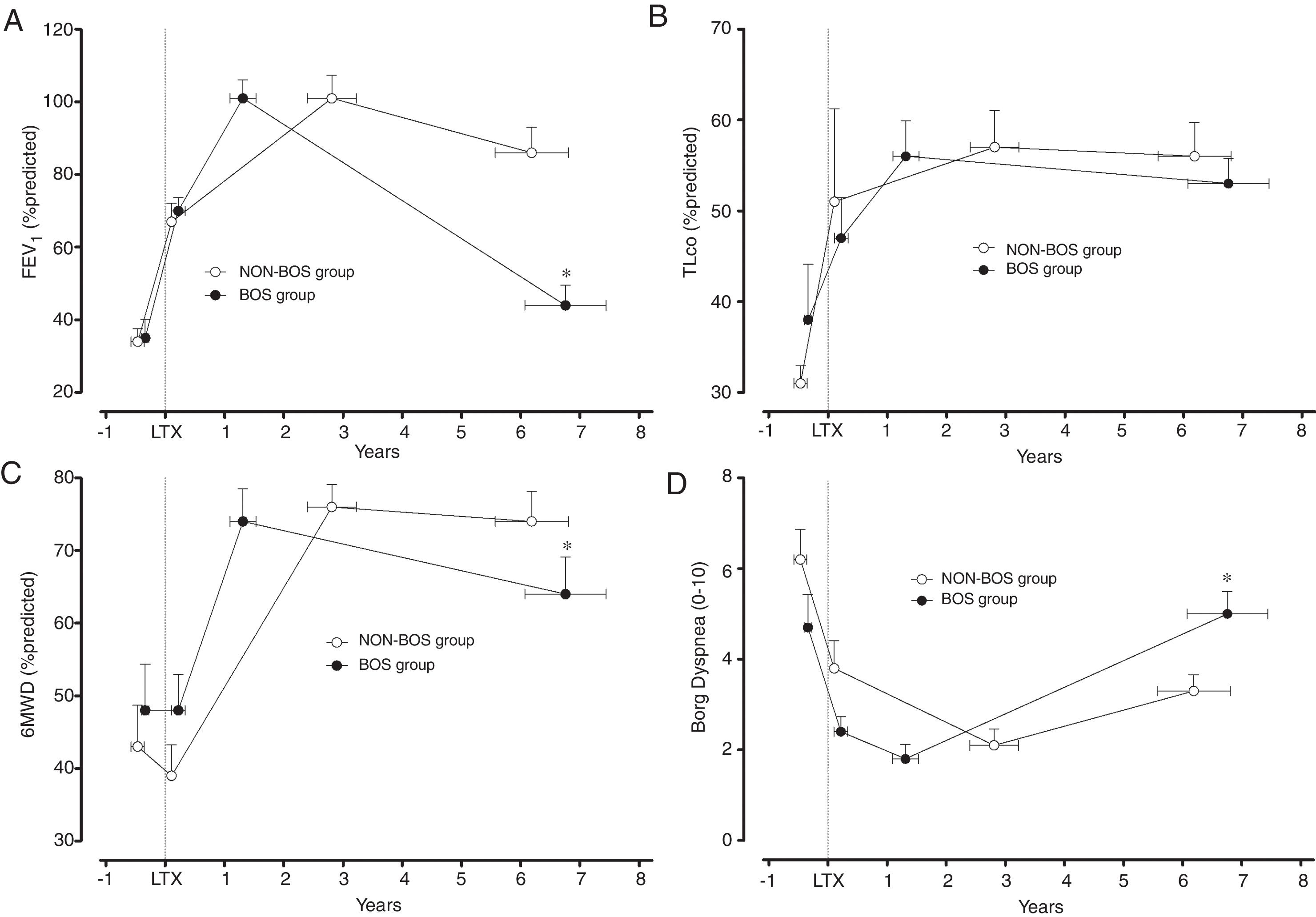
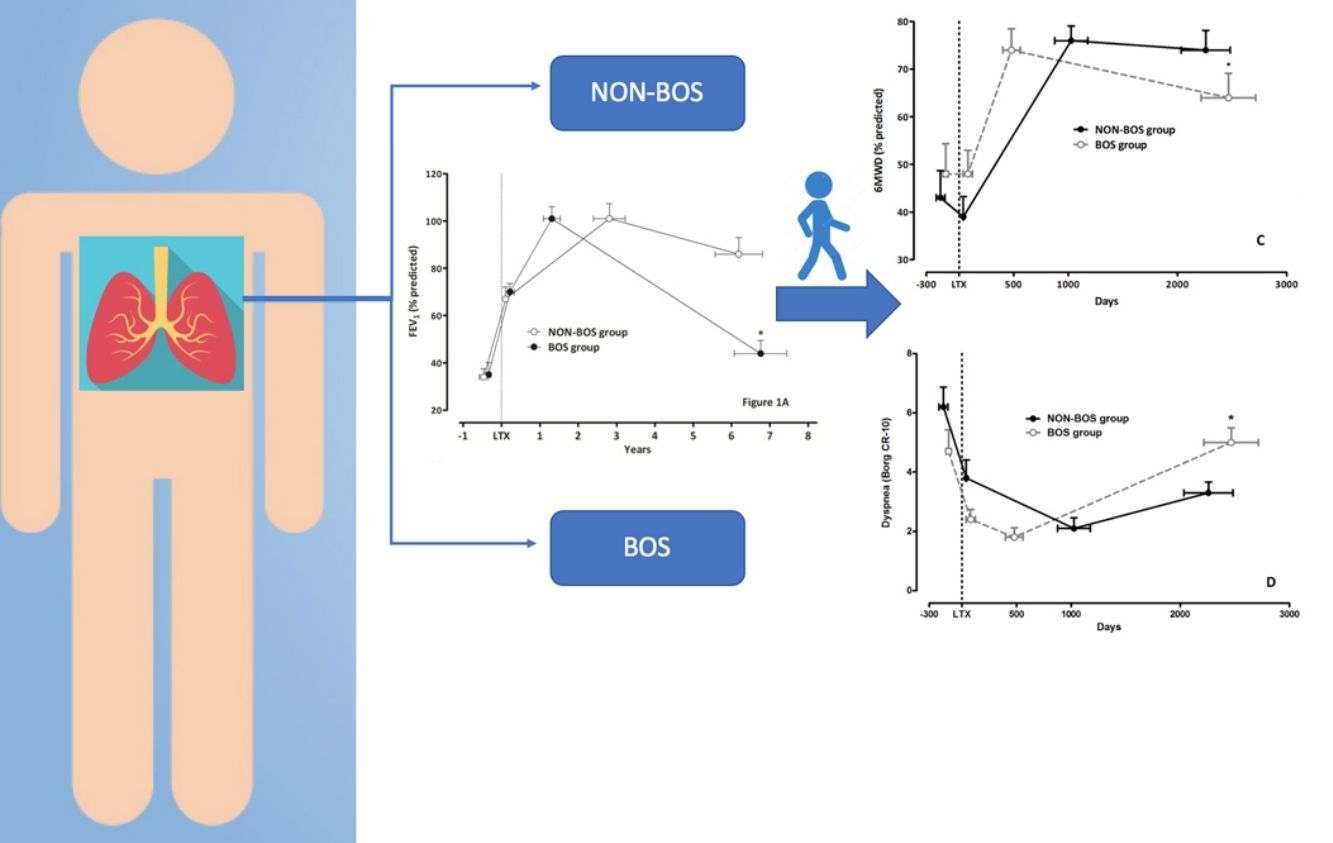
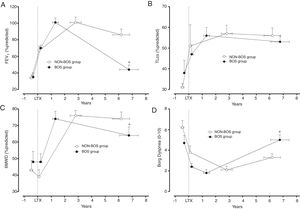
Clinical evolution of patients who developed bronchiolitis obliterans (BOS group) and patients who did not develop bronchiolitis obliterans (NON-BOS group) after lung transplantation. LTx=date of lung transplantation. Symbols reflect measurements taken at 4 different time-points: 1st circle: before LTx (pre-LTx); 2nd circle: immediately after hospital discharge following LTx (post-LTx); 3rd circle: at the follow-up visit when the highest forced expiratory volume in one second (FEV1) was measured (peak FEV1); and 4th circle: at the most recent visit (recent value); *statistically significant difference between groups (p<0.05).
Both groups presented a progressive improvement in FEV1 and FVC in the first months after LTx before achieving similar peak pulmonary function values (FEV1 101±31% predicted vs. 101±25% predicted, p=0.96; FVC 102±29% predicted vs. 101±19% predicted, p=0.80; in the NON-BOS and BOS group, respectively; Fig. 1A and Table 2). After achieving peak FEV1 the BOS group experienced a faster deterioration of pulmonary function as illustrated by significant differences in the evolution of pulmonary function variables (FVC and FEV1) over time between groups (p<0.001 and p=0.001 for group*time interaction of FEV1 and FVC respectively; Table 2). In addition, results of GLM analysis indicate differences between groups at the most recent assessment for both FEV1 (p<0.001; Fig. 1A) and FVC (p=0.013; Table 2). No differences were found in the evolution of TLCO between groups and also not at the most recent assessment (p=0.97; Fig. 1B).
In contrast to the pulmonary function variables and in accordance with previous findings the 6MWD immediately post-LTx was not increased (Table 2). It did only increase in the months following hospital discharge after LTx. The highest 6MWD (% predicted) was achieved close to the peak FEV1 measurement and was similar between groups (76±15% predicted in NON-BOS vs. 74±20% predicted in BOS, p=0.65). Evolution of 6MWD showed a significantly larger decline in BOS patients (group*time interaction: p=0.029; Table 2). Results of GLM analysis further revealed a significant difference in 6MWD between groups at the most recent assessment (p=0.039; Fig. 1C).
No differences between groups were observed in dyspnea symptoms at the peak FEV1 time point (p=0.54; Table 2). The further evolution of dyspnea scores was different between groups (group*time interaction: p=0.024; Table 2). Comparisons with unpaired t-tests revealed a statistically significant difference in dyspnea symptoms between groups at the most recent assessment (p=0.011; Fig. 1D). Evolution of Borg leg fatigue scores was not significantly different between groups (group*time interaction p=0.310; Table 2).
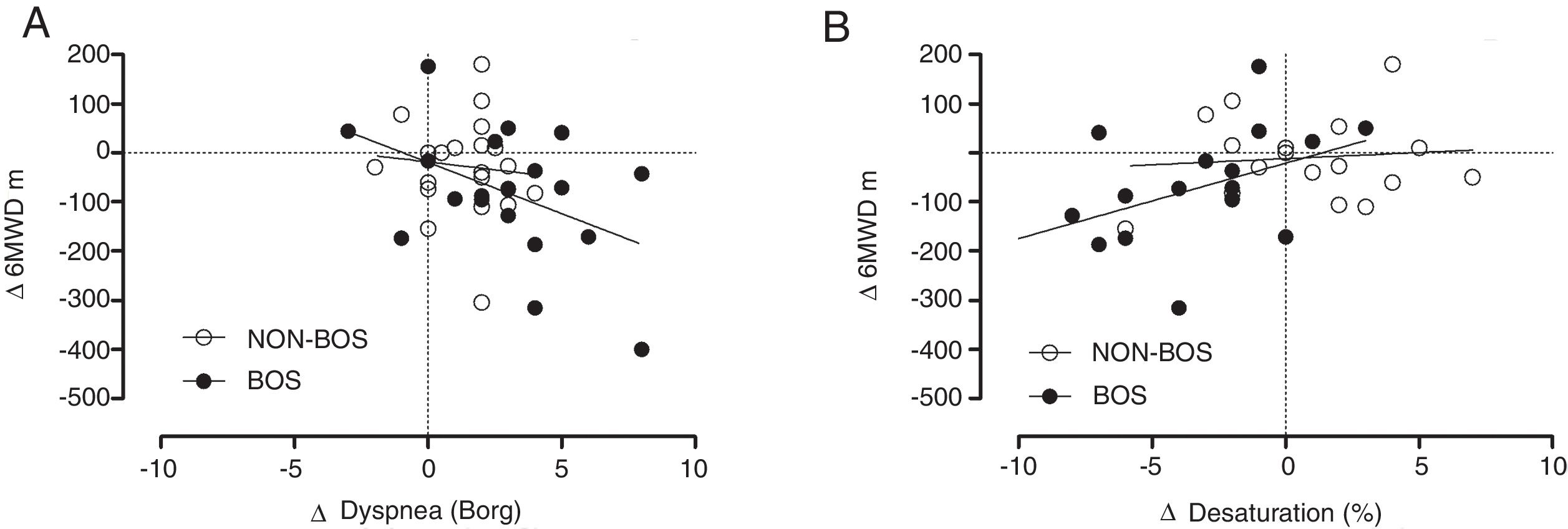
In patients with BOS both changes in dyspnea symptoms and changes in oxygen desaturation during 6MWD (calculated as differences between assessments performed at peak FEV1 assessment and values recorded at the most recent assessment) were significantly correlated with changes in 6MWD (m) (r=−0.447, p=0.048 and r=0.469, p=0.042, respectively) whereas this was not the case in NON-BOS patients (Fig. 2A and B). Changes in leg fatigue symptoms were not significantly correlated with changes in 6MWD (r=−0.194, p=0.219).
Panel (A) changes in reported dyspnea symptom scores (Borg CR-10) plotted against changes in six-minute walking distance (6MWD) in meters; panel (B) changes in peripheral oxygen saturation (SpO2) plotted against changes in six-minute walking distance (6MWD) in meters. Δ=differences between values collected at the follow-up visit when the highest forced expiratory volume in one second (FEV1) was measured (peak FEV1) and values collected at the most recent assessment.
In this retrospective, longitudinal case–control study the functional evolution of LTx recipients with and without BOS diagnosis were compared. Reductions in functional exercise capacity, which were correlated with worsening dyspnea symptoms on exertion, were observed in patients after BOS diagnosis compared with matched patients who did not develop BOS. To our knowledge this is the first longitudinal case–control study showing that patients with BOS after LTx develop significant reductions in FEC and worsening of symptoms.
Our findings are not in agreement with previous observations of stable 6MWD during long-term follow-up in patients developing BOS.7 These different observations might be explained by several factors. First, in the current study all included subjects (n=48) had a complete follow-up after transplantation up to the final assessment. This is in contrast to the study of Gerbase and colleagues in which only 25 of the 58 included patients completed follow-up measurements.7 Patients who dropped out might have been more severely impaired in terms of pulmonary function or functional exercise capacity. This ‘healthy survivor effect’ might have caused the impression of stable 6MWD in the long-term follow-up. By restricting our sample to patients with complete follow-up, we prevented survival bias to influence our results. The larger number of subjects who completed follow-up further increased our ability to detect statistically significant differences between groups. The attainment of about 70–75% of predicted normal FEC at the attainment of peak FEV1 observed in our study is in line with previous findings reporting persisting limitations in exercise capacity after transplantation despite a normal pulmonary function.15–17 In agreement with data from Belloni et al. we observed an initial general increase in FEV1 over time, as well as a concurrent decline of FEV1 and FVC in the BOS group following peak pulmonary function.18 Further support for the validity of our findings (reductions in FEC and increasing symptoms of dyspnea with worsening expiratory flow limitation in the patients who develop BOS) comes from comparisons of our data with observations made in patients with chronic expiratory flow limitation. In patients with COPD, the degree of expiratory flow limitation has consistently been shown to be related to the degree of impairments in functional exercise capacity, and symptoms of dyspnea.19–22 Based on the magnitude of increase in airflow obstruction observed in our BOS cohort (decrease in FEV1 from normal values at peak FEV1 to an average of 44% of predicted normal values after developing BOS) increases in symptoms and reductions in exercise capacity should be expected. The magnitude of observed changes seems physiologically plausible when comparing with data available in patients with chronic expiratory flow limitation.22 It had previously been hypothesized that maintenance of walking capacity in BOS patients might have be achieved by encouraging patients to maintain an active lifestyle. Based on previous data in patients after LTx it seems, however, unlikely that these patients (especially those who develop increasing symptoms of dyspnea due to worsening expiratory flow limitation) will maintain sufficiently physically active.7 Previous studies in LTx recipients with normal pulmonary function revealed that patients remained inactive in comparison to sedentary healthy controls.16,23 Reduced participation in daily activity despite of normal pulmonary function in this patients was strongly associated with remaining impairments in exercise capacity.16,23 The additional dyspnea symptoms developing in BOS patients are likely contributing to further inactivity in this already sedentary group of patients. Again in patients with chronic airflow obstruction it has previously been demonstrated that reductions in participation in physical activity are related to increases in symptoms of dyspnea.22
In patients with normal pulmonary function after LTx peripheral muscle dysfunction contributes importantly to exercise limitation and leg fatigue is usually the main symptom reported by patients to limit exercise, rather than dyspnea.17,24,25 This is in sharp contrast to the situation before LTx where respiratory factors importantly contribute to exercise limitation and dyspnea is usually reported as the main symptom limiting exercise.26 These trends were also observed in the evolution of symptoms in our groups over time (Table 2). While both groups report higher dyspnea scores than leg fatigue scores pre-LTx, they score higher on symptoms of leg fatigue than dyspnea after LTx at the end of a walking test. Again, in patients who develop BOS this trend is reversed at the most recent assessment. Dyspnea symptoms at this point were significantly higher than in NON-BOS patients (Table 2) and were even exceeding their scores before transplantation (Fig. 1D and Table 2). Evolution of weight and BMI were similar between BOS and NON-BOS patients during the follow-up and can therefore be excluded as possible confounding factors for the evolution of FEC in the two groups. The exertional oxygen desaturation that was observed in BOS-patients might also have contributed to the decline in walking capacity (Table 2 and Fig. 2B).
Clinical implicationsAn important limitation of the present study is that both limb muscle function and participation in daily physical activities were not measured. It is well known that both are reduced in patients following LTx and strongly associated with variations in FEC.15,21,27 Increasing symptoms of dyspnea in BOS patients in addition to the already present limb muscle dysfunction are likely contributing to further increases in sedentary behavior, resulting in further deconditioning and reductions in exercise capacity. Exercise training interventions might be considered as a treatment for these patients in order to disrupt this cycle of deconditioning. Pulmonary rehabilitation has been proven to be a very effective intervention to improve symptoms, exercise capacity and muscle function in patients with exertional dyspnea and reduced exercise capacity in various chronic respiratory conditions. In addition, three cohort studies in the long-term post-transplant phase (>12 months post-transplant) all demonstrated positive effects of exercise training on limb muscle function and exercise capacity in LTx recipients.28–30 It was also observed that LTx recipients who were only encouraged to become physically active (in the acute post-LTx phase) remained less active in daily life than those patients who participated in a general exercise training program.23 Tran and colleagues found improvements in 6MWD, subjective symptoms of dyspnea and exercise tolerance in patients with BOS after Hematopoietic Stem Cell Transplantation following an 8-weeks program of Pulmonary Rehabilitation.31 With regard to LTx patients, Fakhro et al. (2017)32 pointed out that those recipients with higher 6MWD, risk for death or Re-LTx is significantly lower, as well as the incidence of developing BOS Grade ≥2. These data support the hypothesis that exercise training could be a useful intervention in BOS patients to reduce symptoms and improve exercise capacity and participation in daily activities.33,34 To test this hypothesis this intervention would need to be prospectively studied both in patients with early diagnosis and those who are waiting for a re-transplant,35 in which the physical condition has been shown to be a predictor of survival.3,32,36 The role of pre-LTx 6MWD as a predictor of BOS development after lung transplant is unclear, our results did not find a significant difference in 6MWD between BOS and NON-BOS groups. In addition there is also a lack of descriptive data on the impact of pulmonary and limb muscle function impairments on limitations in exercise capacity and participation in daily activity in these patients.
To our knowledge this is the first longitudinal case–control study showing that patients developing BOS after LTx develop significant reductions in FEC and worsening of dyspnea symptoms in comparison to patients without BOS. In contrast to previous work, the current study design precluded survival bias to impact on the results. The relationship between BOS, shortness of breath, participation in daily activities, and their impact on FEC and peripheral muscle function needs to be further explored. The effect of exercise training interventions on physical functioning should be studied in order to find out whether rehabilitation might be a useful treatment option for these patients.
Conflict of interestThe authors declare no conflict of interests.
We would like to thank the complete staff of the lung transplant and pulmonary rehabilitation units of the University Hospital Leuven for their support of the project. We would also like to express our gratitude to the patients for providing consent to use their clinical data for research purposes.