CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
More infoEosinophil-associated lung disease often occurs following certain viral infections and is a known complication of past vaccinations for severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and other viruses.1 However, to date no similar complications have been reported in response to SARS-CoV-2 vaccination. The authors present two unusual cases of eosinophilic pneumonia following SARS-CoV-2 vaccination.
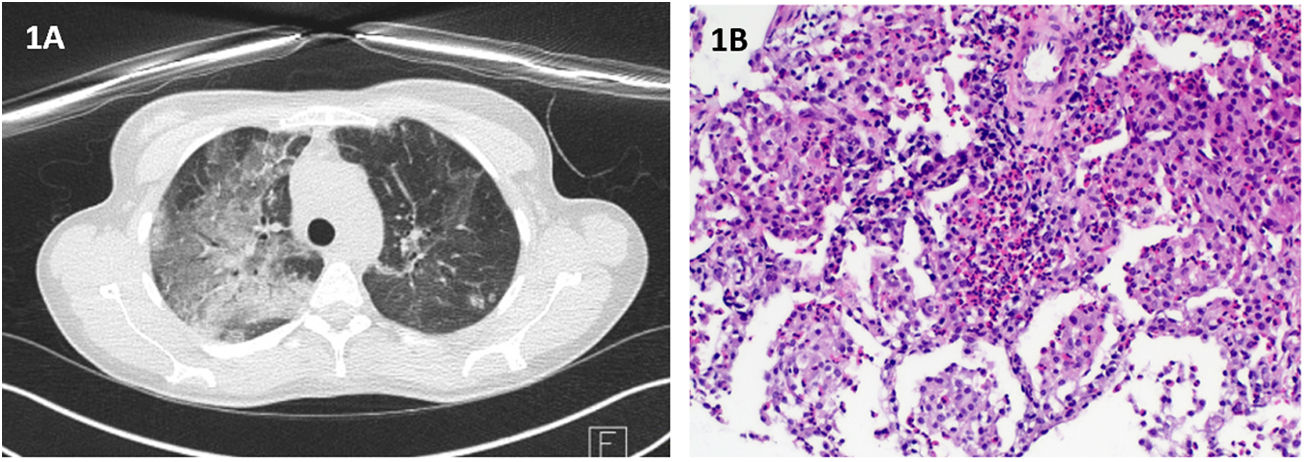
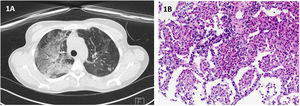
Case 1: A 38-year-old Caucasian female, former smoker, without relevant medical history nor chronic treatment, presented at consultation with a 1-month progressive dyspnea, aggravated during the last week, and dry cough, pleuritic chest pain and an isolated episode of fever (38°C). Initial workup revealed blood eosinophilia (23.8%; 3250/μL) without neutrophilia and increased C-reactive protein (37.7mg/dL) and total IgE (420). A course of Levofloxacin was started. Of note, symptoms initiated two weeks after COVID-19 vaccination. Physical examination was unremarkable. PCR for SARS-CoV-2 was negative. Chest radiography showed airspace consolidation in the right upper lobe. Chest HRCT revealed multiple ground glass opacities and interlobular septal thickening (Fig. 1A). Flexible bronchoscopy with BAL analysis revealed eosinophilia (13%) and lymphocytosis (15%). All microbiological and immunological tests were negative. Rearrangement of PDGFRA was assessed and not found. CT-guided transthoracic lung biopsy was suggestive of eosinophilic pneumonia (Fig. 1B). Deflazacort 60mg/day was started, with both clinical and radiological resolution.
(A) Chest HRCT: multiple ground glass opacities and interlobular septal thickening; (B) Histopathological findings of transthoracic needle biopsy: On hematoxylin and eosin staining there is lung tissue with alevoli filled by histiocytes and eosinophils, forming “eosinophilic microabscesses”. (Original magnification: 100×).
Case 2: Forty-seven-year-old caucasian female, non-smoker, with history of psoriasis (treated with ustecinumab since 2019) and depression (treated with fluoxetine 20mg/day). She had a history of nonresolving pneumonia in 2009, at that time with documented blood eosinophilia; diagnostic work-up was non-conclusive and spontaneous resolution of symptoms and radiological changes were observed. Regular follow-up did not show clinical/radiological recurrence.
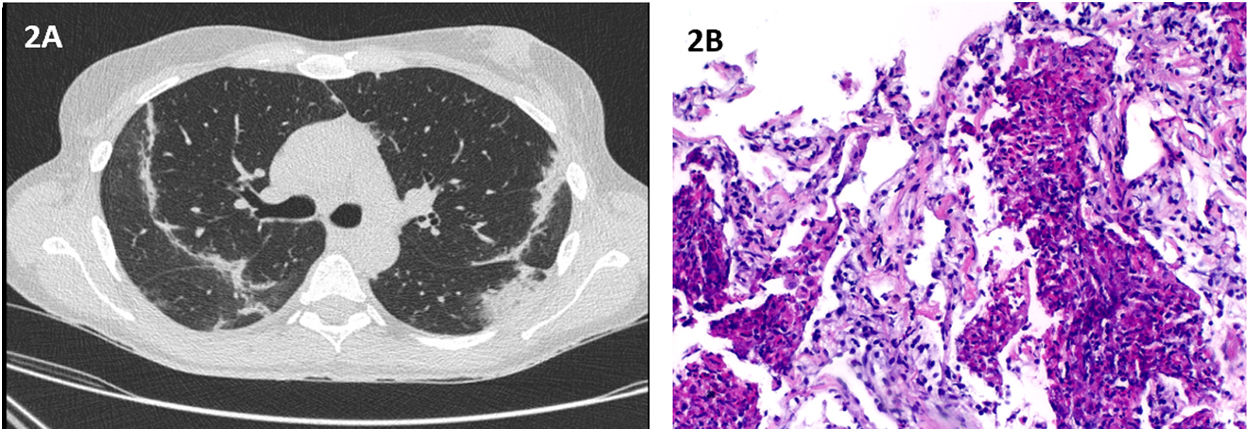
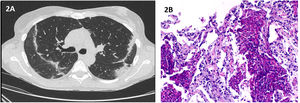
In July 2021, she presented at the emergency department complaining of fatigue and troublesome dry cough for the last 3 weeks. A course of claritromicin was completed, with no symptomatic improvement. Physical examination revealed crackles in the upper lung fields. Initial workup revealed peripheral eosinophilia (10.1–820/μL), with no leukocytosis and normal C-reactive protein. Patient was administered with SARS-COV-2 vaccine four weeks before symptoms appeared. Chest HRCT revealed peripheral and subpleural areas of linear parenchymal densification (Fig. 2A). Flexible bronchoscopy with BAL analysis revealed eosinophilia (11%). All microbiological and immunological studies were normal, with exception of positive antinuclear antibodies 1/320 with speckled pattern. CT-guided transthoracic lung biopsy was consistent with eosinophilic pneumonia (Fig. 2B). The patient was started on prednisolone 40mg/day, with clinical and radiological resolution at the end of 14 days.
(A) Chest HRCT: peripheral and subpleural areas of linear parenchymal densification; (B) Histopathological findings of transthoracic needle biopsy: Besides the “eosinophilic microabscesses” there is also more evident alveolar thickening, edema and foci of fibrosis. (Original magnification: 100×).
Acute eosinophilic pneumonia (AEP) is a rare disease that can be idiopathic or secondary to innumerous agents.2 The type of T-helper immune response induced by vaccination depends on the antigen (e.g. immunization with inactivated SARS-CoV-1; Immunization with the whole spike (S) protein).3 In the past SARS-CoV-1 vaccines have been shown to induce pulmonary eosinophilia in animals after viral challenge3 and eosinophil-associated type 2 inflammation in reinfection in monkeys.3 Eosinophil associated pulmonary disease was also seen subsequently to infection after RSV vaccination3 and a case of AEP related to influenza vaccination has also been reported.4
Moreover, cases of AEP in patients with COVID-195 or expressed as a recurrence of respiratory symptoms after COVID-19 recovery were documented.2
Since SARS-CoV-1 and SARS-CoV-2 share more than 80% identity,1 it would be no surprise if SARS-CoV-2 vaccines could cause a similar vaccine-associated immunopathology.
In both cases, possible causes of eosinophilic pneumonia, including parasitic infestation, drug-induced eosinophilia, and dust or toxic substance exposure were excluded, therefore the positive temporal relationship between SARS-CoV-2 vaccination and symptoms’ emergence lead the authors to believe that vaccination might be the potential cause of AEP.
With this report, the authors intend to highlight the potential development or recrudescence of eosinophilic lung disease in association with SARS-CoV-2 vaccination.
Conflicts of interestThe authors declare that they have no conflicts of interest.