In recent years, the need to diagnose and treat ever smaller lung lesions or nodules with a limited solid component has increased. Multiple endoscopic and image-guided diagnostic techniques are now at our disposal, but when these methods fail, the next step is diagnostic-therapeutic, preferably minimally invasive, surgery. However, the development of these techniques has contributed to an increased difficulty in locating small lesions. Finally, the need to repeat biopsies of previously diagnosed lesions also complicates their localization for surgery.
The objective of this study was to evaluate the feasibility and safety of using a radiolocalization technique for labeling hidden lung lesions.
Between September 2014 and September 2020, 45 patients from our hospital with pulmonary lesions suggestive of malignancy were selected consecutively and prospectively. Patients with lesions showing progressive growth on successive computerized tomography (CT) scans or pathological uptake on 18-fluorodeoxyglucose positron emission tomography-CT (PET-CT) were included. Lung lesions had to present any of the following characteristics: lesion less than 1 cm, subsolid lesion, or lesion at a depth of more than 1 cm. Lesions with a solid component measuring more than 2 cm were excluded. All cases were presented to the multidisciplinary oncology committee, who discussed the clinical case, need for surgical biopsy, and the possibility and feasibility of performing a CT-guided radiotracer labeling.
A lesion was defined as pure ground glass or lacking in solid component when it disappeared completely in the mediastinal window of the CT1. Lesions were defined as subsolid if the nodular component that remained visible in the mediastinal window of the CT was greater than 6 mm1.
The lesion was labeled within 2 h before the intervention. The selected radiotracer was 99TC-labeled macroaggregate albumin (LyoMAA Technoscan® 2 mg, Curium, Netherlands) with a half-life of 2−8 h and a particle size of 10−100 μm. Prior to injection of the tracer, local anesthesia consisting of 10 ml mepivacaine 1% was applied at the thoracic puncture site. A transthoracic puncture using a 22 G needle guided by CT without intravenous contrast material was then performed in or near the lesion. After checking the correct position of the needle with new CT slices, the radiotracer was injected. After injection, new slices were obtained to check for the absence of complications, such as pneumothorax or bleeding. Crossing of fissures and damage to pulmonary bullae were avoided during the puncture. After the procedure, the patient was transferred to the nuclear medicine unit, where a single photon emission computed tomography-CT (SPECT-CT) was performed (Discovery NM/CT 670®, GE Healthcare, Boston, US) to check that the tracer was positioned correctly, had not migrated to nearby areas, and was sufficiently active.
During surgery, the lesion was located using a detector probe (Navigator GPS®, RMD Instruments, US) and pulmonary resection was performed according to the standard technique. The approach route was selected according to the location and depth of the lesion and the presence of other lung lesions requiring palpation. On completion of resection, the lack of residual uptake at the resection margins (<10% of the peak) was confirmed and the specimen was sent to the pathology department. In patients whose lesion was consistent with lung carcinoma and who tolerated the procedure, the intervention was completed with anatomical lung resection.
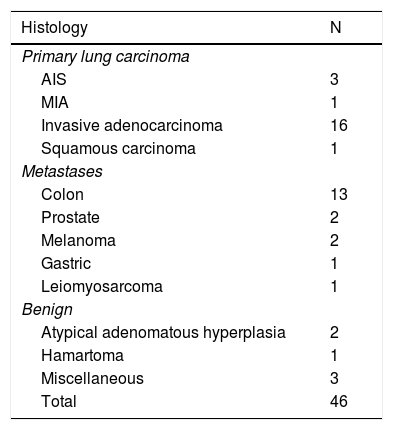
Intra-surgical labeling and radiographic localization were performed in 46 nodules from 45 patients. The procedure was ruled out in 1 patient because of accidental aortic puncture. Mean age was 65.69 ± 7.14 years, 32 were men and 13 women. The mean size of the nodules was 8.8 ± 4.00 mm (range 0.6–20 mm) and the mean distance to the nearest pleura was 15.37 ± 14.39 mm (range 2–68 mm). The morphology of the nodules was ground glass, semi-solid, and solid in 22 (47.82%), 6 (13.04%), and 18 nodules (39.13%), respectively. Pathology findings are listed in Table 1. Fifteen patients underwent minimally invasive surgery, 27 thoracotomy, and 3 rethoracotomy. Two patients had affected margins: 1 was re-operated and another completed treatment with radiation therapy. Thirteen patients had post-puncture pneumothorax (28.8%), but none required pleural drainage. The only complication during the procedure was accidental aortic puncture that required no intervention due to the absence of bleeding after the event. The right upper lobe (34.78%, n = 16) was the most frequent site, followed by right lower lobe (32.6%, n = 15), left upper lobe (15.21%, n = 7), left lower lobe (13.04%, n = 6) and middle lobe (4.34%, n = 2).
Histology of pulmonary nodules.
| Histology | N |
|---|---|
| Primary lung carcinoma | |
| AIS | 3 |
| MIA | 1 |
| Invasive adenocarcinoma | 16 |
| Squamous carcinoma | 1 |
| Metastases | |
| Colon | 13 |
| Prostate | 2 |
| Melanoma | 2 |
| Gastric | 1 |
| Leiomyosarcoma | 1 |
| Benign | |
| Atypical adenomatous hyperplasia | 2 |
| Hamartoma | 1 |
| Miscellaneous | 3 |
| Total | 46 |
AIS: adenocarcinoma in situ; AMI: minimally invasive adenocarcinoma.
Digital palpation has historically been the reference technique for locating lung lesions. However, more than half of lesions measuring less than 10 mm located at a depth of more than 5 mm can go unnoticed2. Several techniques have been developed for locating these lesions and performing minimally invasive surgery, bypassing the need for digital palpation. The most important of these are hook wire labeling, methylene blue labeling, ultrasound-guided intraoperative localization, and the most modern, radiolocalization.
Hook wire labeling helps localize up to 97% of the lesions, although the wire can move in up to 48% of cases3. Gonfiotti compared the use of hook wires with radiolocalization and found no statistically significant differences (84% vs 96%). Both methods were superior to digital palpation, which located only 24% of lesions4. The use of other techniques, such as endothoracic ultrasonography5 or labeling by injecting contrast agents such as methylene blue6, have also been described. The main advantage of ultrasonography is the absence of complications7, but its use is limited by interoperator variability and diagnostic difficulties in very emphysematous parenchyma.
In conclusion, radioguided localization of hidden lesions is, in our experience, a safe and effective technique that is useful in the resection of small, intraparenchymal lesions, achieving adequate margins using conventional or minimally invasive surgery.
Please cite this article as: Garcia-Reina S, Fernández E, Lafuente Carrasco S, Margelí V, Gómez C, Moragas G, et al. Eficacia de la localización radioguiada para el marcaje de lesiones pulmonares quirúrgicas ocultas. Arch Bronconeumol. 2021;57:711–712.