The impact of pulmonary hypertension (PH) on exercise tolerance in chronic obstructive pulmonary disease (COPD) has not been fully elucidated. It is necessary to characterize pulmonary hemodynamics in patients with moderate to severe COPD in order to improve their management. The aim of the study was to determine whether in COPD the presence of PH is associated with reduced exercise tolerance in a cohort of stable COPD patients.
MethodsCross-sectional analysis of 174 COPD patients clinically stable: 109 without PH and 65 with PH (COPD-PH). We assessed socio-demographic data, lung function, quality of life, dyspnea, cardiopulmonary exercise testing (CPET), constant workload endurance time (CWET), and six-minute walk test (6MWT). We elaborated a logistic regression model to explore the impact of PH on exercise capacity in COPD patients.
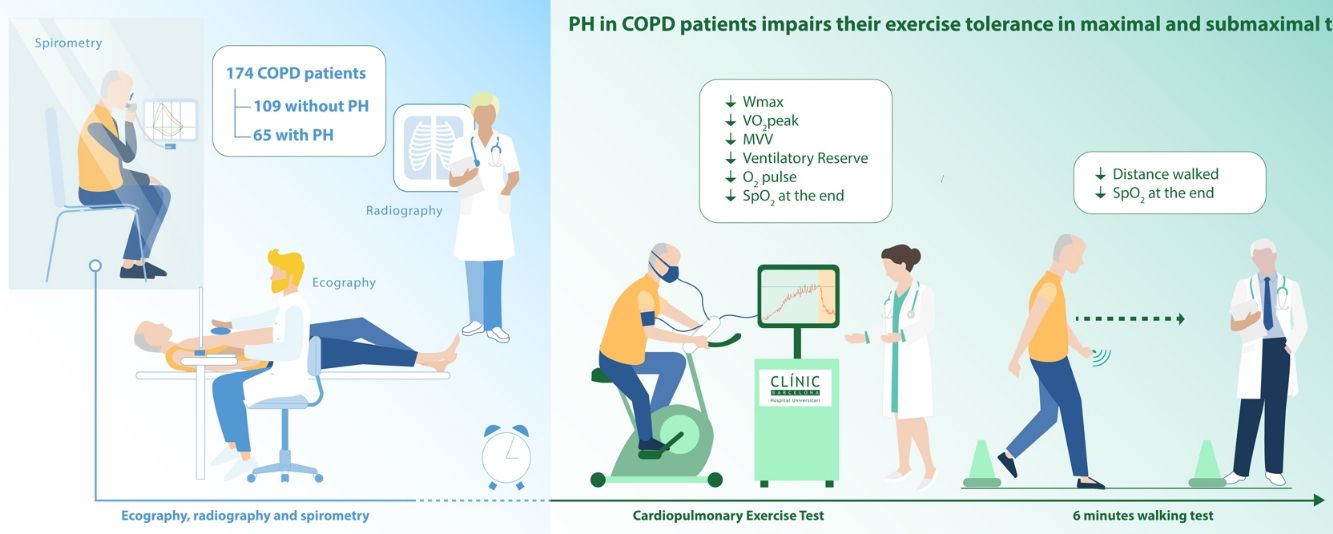
ResultsCOPD-PH patients showed lower exercise capacity both at maximal (CPET) (43(20) versus 68(27) Watts and 50(19)% versus 71(18)% predicted peak oxygen consumption (VO2peak), COPD-PH and COPD, respectively), and at submaximal tests (6MWT) (382(94) versus 486(95) m). In addition, the COPD-PH group had lower endurance time than the non-PH COPD group (265(113) s and 295(164) s, respectively).
ConclusionsThe presence of PH is an independent factor that impairs exercise capacity in COPD.
El impacto de la hipertensión pulmonar (HTP) en la tolerancia al ejercicio en la enfermedad pulmonar obstructiva crónica (EPOC) no se ha dilucidado en su totalidad. Es necesario caracterizar la hemodinámica pulmonar de los pacientes con EPOC moderada a grave para poder mejorar su manejo. El objetivo de este estudio fue determinar si la presencia de HTP en la EPOC se asociaba con una disminución en la tolerancia al ejercicio en una cohorte de pacientes con EPOC estable.
MétodosEstudio transversal de 174 pacientes con EPOC clínicamente estables: 109 de ellos no mostraban HTP y 65 de ellos sí (EPOC-HTP). Valoramos la información sociodemográfica, la función pulmonar, la calidad de vida, la disnea, realizamos una prueba de ejercicio cardiopulmonar (PECP), medimos el tiempo de tolerancia de ejercicio constante y realizamos de marcha de seis minutos (6MWT, por sus siglas en inglés). Elaboramos un modelo de regresión logística para explorar el impacto de la HTP en la capacidad de ejercicio de los pacientes con EPOC.
ResultadosLos pacientes con EPOC-HTP mostraron una menor capacidad de ejercicio, tanto en las pruebas máximas (PECP) (43(20)W frente a 68(27)W y 50(19)% frente a 71(18)% de consumo de oxígeno máximo predicho (VO2max), para pacientes con EPOC-HTP y pacientes con EPOC, respectivamente) como en las pruebas submáximas (6MWT) (382(94)m frente a 486(95)m). Además, el grupo de EPOC-HTP presentó un menor tiempo de resistencia que el grupo de EPOC sin HTP (265(113)s y 295(164)s, respectivamente).
ConclusionesLa presencia de HTP es un factor independiente que afecta a la capacidad de ejercicio en la EPOC.
Pulmonary hypertension (PH) is a common complication of chronic obstructive pulmonary disease (COPD).1 The prevalence of PH in COPD patients it is not negligible, it has been reported from 23% to 91%, depending on the severity of the disease and the diagnostic criteria used to define it.2–5 Clinically, PH increases the risk of hospitalization for COPD exacerbation and entails a lower survival rate.3,6
Patients with COPD typically show reduced exercise tolerance compared with healthy controls with similar anthropometric characteristics. Several causal factors have been proposed. On one hand, increased ventilatory requirements and abnormal dynamic ventilatory mechanics7 are thought to be the main limiting factor, although peripheral muscle weakness8 and right ventricular diastolic function9 have also shown to limit exercise capacity in COPD patients. Moreover, the presence of PH has been hypothesized to further decrease exercise tolerance.3,10 It is necessary to characterize pulmonary hemodynamics in patients with moderate to severe COPD-PH in order to improve their management and to choose appropriate rehabilitation strategies.
Several studies have attempted to clarify whether the exercise limitation in COPD patients with PH is primarily due to the ventilatory impairment or to the vascular component with contradictory results. Sims et al.10 found that in patients with severe COPD the presence of PH was associated with lower distance covered in the six-minute walk test (6MWT), regardless of pulmonary function, anthropometric or demographic characteristics. Also, they showed that higher pulmonary arterial pressure (PAP) was associated with lower exercise capacity. More recently, the presence of PH was found to worsen maximal exercise capacity in a group of severe COPD patients.11 However, there are also opposite studies suggesting that exercise impairment is due, basically, to the ventilatory limitation.12–15 The majority of previous studies present some limitations, such as a selective sample only including severe COPD patients candidates for lung transplantation,12 small sample size14 or inaccurate definition of PH,15 that might complicate getting clear conclusions.
The 6th World Symposium on Pulmonary Hypertension (WSPH) “encourage cardiopulmonary exercise test (CPET) for a more elaborate distinction between pulmonary versus circulatory limitation” with the aim to discriminate between different groups of PH. Specifically between the group 1 (pulmonary arterial hypertension, PAH) with a predominant cardiovascular profile (exhausted circulatory reserve) versus the group 3 with a predominant obstructive/restrictive profile (exhausted ventilatory reserve).16 This distinction arise from the study by Boerrigter et al.17 who showed that COPD patients with severe PH (mean PAP ≥40mmHg) had an exhausted circulatory reserve at the end of exercise with a reduced slope of the cardiac output/oxygen consumption ratio, low mixed venous oxygen saturation and low arterial partial pressure of CO2 (PaCO2), whilst the breathing reserve was preserved. On the other hand, COPD patients with moderate PH or without PH showed increased PaCO2 and exhaustion of the breathing reserve at the end of the exercise, whereas the circulatory reserve was maintained.17
Therefore, we aimed to determine whether in a cohort of stable COPD patients the presence of PH is associated with reduced exercise tolerance, as assessed by maximal and submaximal exercise tests, and worse quality of life.
MethodsDesign and settingThis was a cross-sectional study comparing baseline data of Caucasian patients with COPD recruited and assessed at the Hospital Clinic (Barcelona, Spain). Briefly, COPD candidates referred to the pulmonary rehabilitation (PR) service of our hospital were screened for PH. Those with PH were included in the SILD-COPD randomized double-blinded controlled trial (NCT 01055405). Patients without PH followed the standard PR program of our Institution. We followed the STROBE guidelines.18 All patients understood, agreed, and signed the informed consent approved by the institutional review boards at Hospital Clinic (Barcelona, Spain).
ParticipantsAdult patients aged between 40 and 80 years with a diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD),1 clinically stable with optimized pharmacological treatment, without a history of lower respiratory tract infection and/or COPD exacerbation within 6 weeks prior to evaluation were assessed for PR.
The presence of associated PH was assessed using Doppler echocardiography in all patients. PAP was considered abnormally increased when the maximum tricuspid regurgitation velocity was greater than 2.7ms−1, which is equivalent to an estimated systolic PAP>34mmHg19,20 calculated using Bernoulli's equation, assuming a right atrial systolic pressure of 5mmHg.21 In patients who had been subjected to right heart catheterization (RHC) (n=14), we considered a definitive diagnosis of PH when mean PAP was ≥25mmHg.19 We only included patients with PH from group III, meaning PH due to lung diseases and/or hypoxia. Other potential causes of PH, such as chronic thromboembolic pulmonary hypertension or left heart disease (systolic function and valve diseases), were discarded, as well as the previous history of ischemic or mitral or aortic valve diseases.
EvaluationThe following descriptive variables were assessed: socio-demographic data (age, sex, smoking status), lung function (forced spirometry, lung volumes, diffusing capacity of the lungs for carbon monoxide (DLCO) and arterial blood gases breathing room air). Also, health-related quality-of-life was assessed by the Spanish version of the St George's Respiratory Questionnaire (SGRQ),22 and dyspnea symptoms were measured by the modified Medical Research Council (mMRC) dyspnea scale.23
All patients were evaluated with an incremental CPET,24 a constant work-load exercise test (to measure endurance time) (CWET), both conducted on a cycle ergometer (Lode Corival CEPT mod: 960900, Groningen, The Netherlands),25 and a 6MWT.26 In order to ensure a reliable endurance time, during the constant work-rate exercise COPD patients with PH performed the test at the 70% of the peak work-load achieved in the incremental test, whereas COPD patients without PH carried out the test at the 80% of the peak work-load. Patients on long-term oxygen therapy were assessed without oxygen during the evaluation tests on the cycle ergometer (PH group, n=18) but performed the 6MWT under oxygen.
StatisticsQuantitative variables were expressed as mean and standard deviation (SD) or median and Interquartile Range (IQR: 25th; 75th percentiles). Qualitative variables were described as frequencies and percentages (n, %). Differences between groups were evaluated using Student's t-test for normally distributed variables, Mann–Whitney U test for non-parametric variables, or Fisher's exact test for comparing proportions.
We estimated the odds ratio (OR) and their 95% confidence intervals (95% CI) from logistic regression model to explore the effect of PH on exercise capacity in COPD patients independently of other variables such as (i) lung function and (ii) socio-demographic characteristics. Other factors were introduced in the analyses were selected based on statistical significance in the univariable analyses, and their potential physiological relationship with the presence of PH. Cut-off points were dichotomized based on the median value of the distribution for distance walked in the 6MWT, peak oxygen consumption (VO2peak) and SGRQ score. In these comparisons, all patients were considered. Different models for the effect of PH adjusted by age, BMI, forced expiratory volume in the first second (FEV1)% predicted, DLCO, partial pressure of O2 in arterial blood (PaO2), mMRC, and SGRQ score were were performed and the results from logistic regression were presented as OR (95% CI). The level of significance was set at the standard two-sided level of 5%. All the analyses were performed using the statistical package SPSS version 25.0 software (IBM Corporation, Armonk, NY, USA).
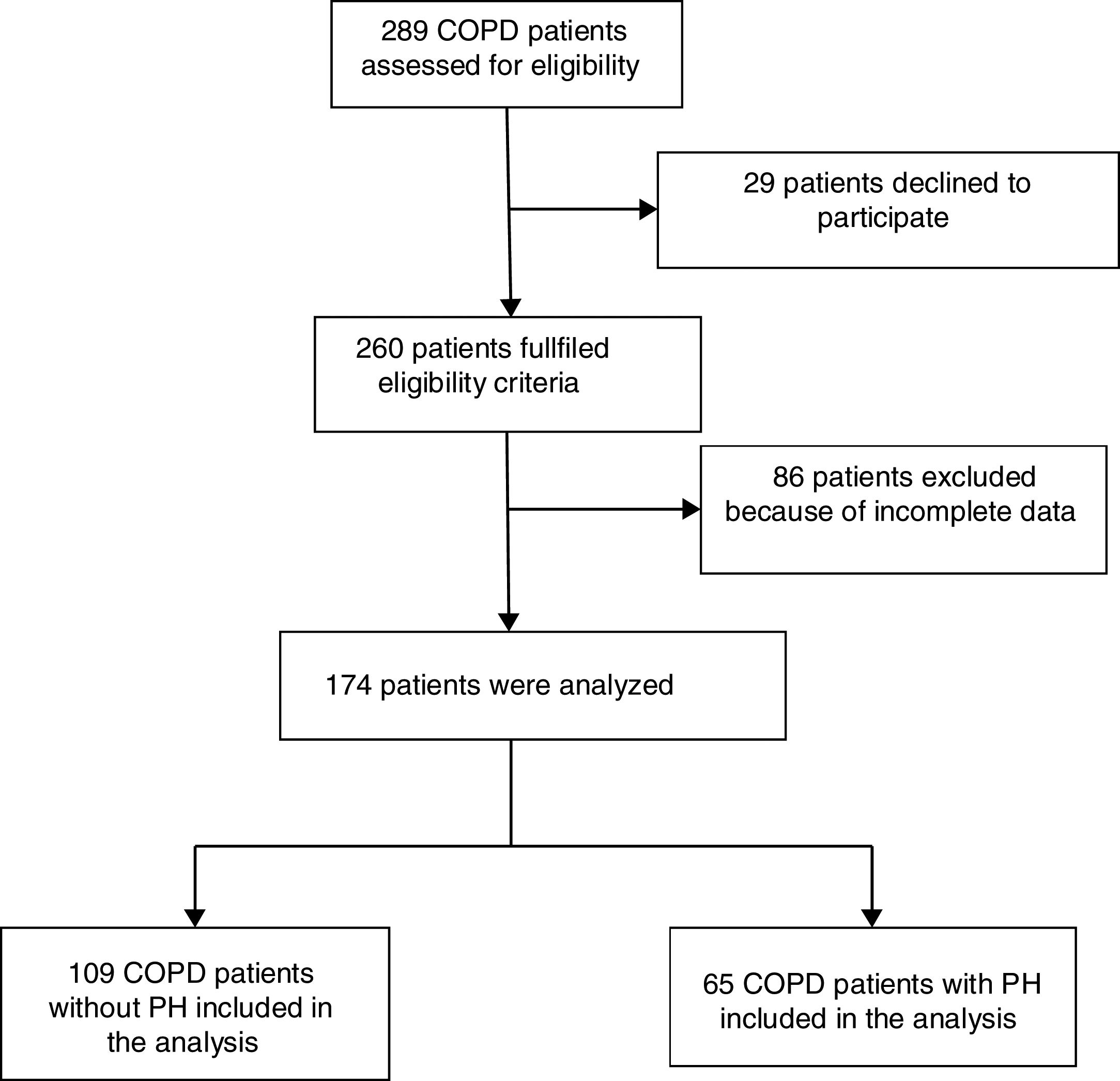
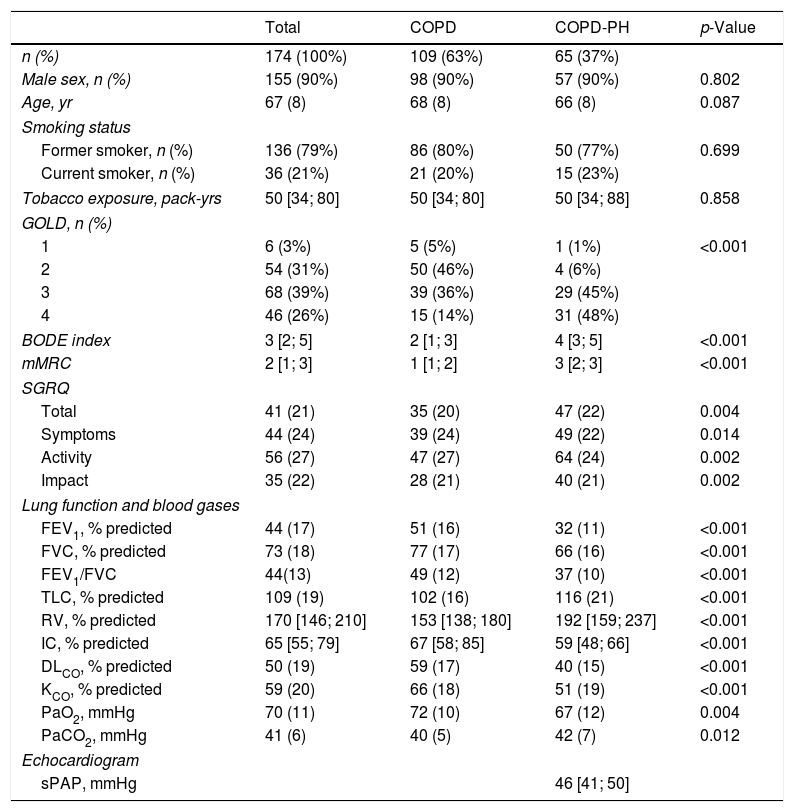
ResultsPatient's characteristicsFig. 1 describes the overall flow-chart of patients. We present data of the 174 patients analyzed who were fully assessed, 109 with COPD without PH (non-PH COPD group): 90% male, 68(8) years old and FEV1 of 51(16)% of predicted value; and, 65 with COPD and associated PH (COPD-PH group): 90% male, 66(8) years old and FEV1 of 32(11)% predicted. Clinical characteristics are displayed in Table 1. All four GOLD stages are presented in both groups; although 48% of patients of the COPD-PH group were classified as GOLD 4, whereas 46% in the non-PH COPD group were classified as GOLD 2. Globally, COPD-PH patients showed worse clinical and lung function characteristics. There was no significant difference in body mass index (BMI) between groups; although, two subjects with PH had a BMI below 18.5kg/m2 (underweight).
Demographic and clinical characteristics of the patients.
| Total | COPD | COPD-PH | p-Value | |
|---|---|---|---|---|
| n (%) | 174 (100%) | 109 (63%) | 65 (37%) | |
| Male sex, n (%) | 155 (90%) | 98 (90%) | 57 (90%) | 0.802 |
| Age, yr | 67 (8) | 68 (8) | 66 (8) | 0.087 |
| Smoking status | ||||
| Former smoker, n (%) | 136 (79%) | 86 (80%) | 50 (77%) | 0.699 |
| Current smoker, n (%) | 36 (21%) | 21 (20%) | 15 (23%) | |
| Tobacco exposure, pack-yrs | 50 [34; 80] | 50 [34; 80] | 50 [34; 88] | 0.858 |
| GOLD, n (%) | ||||
| 1 | 6 (3%) | 5 (5%) | 1 (1%) | <0.001 |
| 2 | 54 (31%) | 50 (46%) | 4 (6%) | |
| 3 | 68 (39%) | 39 (36%) | 29 (45%) | |
| 4 | 46 (26%) | 15 (14%) | 31 (48%) | |
| BODE index | 3 [2; 5] | 2 [1; 3] | 4 [3; 5] | <0.001 |
| mMRC | 2 [1; 3] | 1 [1; 2] | 3 [2; 3] | <0.001 |
| SGRQ | ||||
| Total | 41 (21) | 35 (20) | 47 (22) | 0.004 |
| Symptoms | 44 (24) | 39 (24) | 49 (22) | 0.014 |
| Activity | 56 (27) | 47 (27) | 64 (24) | 0.002 |
| Impact | 35 (22) | 28 (21) | 40 (21) | 0.002 |
| Lung function and blood gases | ||||
| FEV1, % predicted | 44 (17) | 51 (16) | 32 (11) | <0.001 |
| FVC, % predicted | 73 (18) | 77 (17) | 66 (16) | <0.001 |
| FEV1/FVC | 44(13) | 49 (12) | 37 (10) | <0.001 |
| TLC, % predicted | 109 (19) | 102 (16) | 116 (21) | <0.001 |
| RV, % predicted | 170 [146; 210] | 153 [138; 180] | 192 [159; 237] | <0.001 |
| IC, % predicted | 65 [55; 79] | 67 [58; 85] | 59 [48; 66] | <0.001 |
| DLCO, % predicted | 50 (19) | 59 (17) | 40 (15) | <0.001 |
| KCO, % predicted | 59 (20) | 66 (18) | 51 (19) | <0.001 |
| PaO2, mmHg | 70 (11) | 72 (10) | 67 (12) | 0.004 |
| PaCO2, mmHg | 41 (6) | 40 (5) | 42 (7) | 0.012 |
| Echocardiogram | ||||
| sPAP, mmHg | 46 [41; 50] | |||
Definition of abbreviations: BMI: body mass index; mMRC: modified Medical Research Council scale; SGRQ: Sant George Respiratory Questionnaire; FEV1: force expiratory volume in the first second; %pred: % of the predicted value; FVC: force vital capacity; IC: inspiratory capacity; RV: residual volume; TLC: total lung capacity; DLCO: diffusion capacity of the lung for CO; KCO: DLCO corrected for alveolar volume; PaO2: partial pressure of O2 in arterial blood; PaCO2: partial pressure of CO2 in arterial blood; sPAP: systolic Pulmonary Arterial Pressure. Values are expressed as mean (SD) when data is normally distributed or as median and interquartile range [P25–P75] when data distribution is skewed.
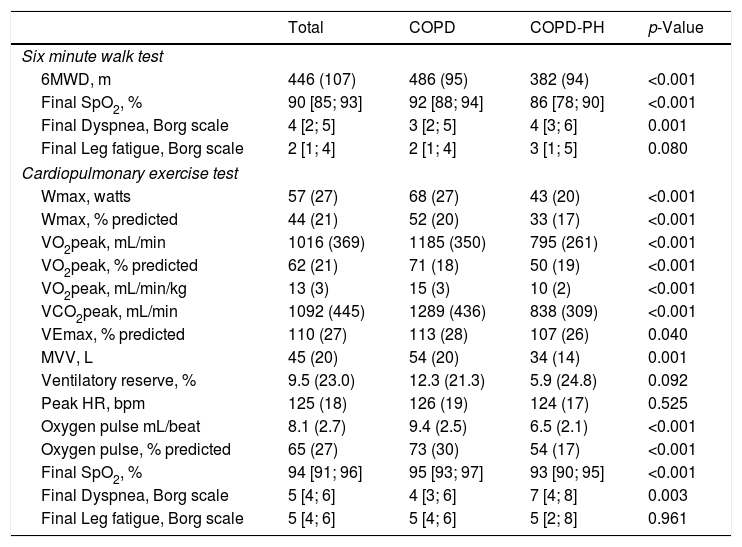
Table 2 shows the response to CPET and 6MWT. COPD-PH patients showed lower exercise capacity both at maximal (CPET) and submaximal (6MWT) tests. In addition, COPD-PH patients had shorter endurance time than the non-PH COPD group (265(113) s, and 295(164) s, respectively) despite being assessed at a 10% lower work-load. The difference in work-load prevented statistical comparison.
Data on exercise capacity measured by different exercise tests (6MWT and CPET).
| Total | COPD | COPD-PH | p-Value | |
|---|---|---|---|---|
| Six minute walk test | ||||
| 6MWD, m | 446 (107) | 486 (95) | 382 (94) | <0.001 |
| Final SpO2, % | 90 [85; 93] | 92 [88; 94] | 86 [78; 90] | <0.001 |
| Final Dyspnea, Borg scale | 4 [2; 5] | 3 [2; 5] | 4 [3; 6] | 0.001 |
| Final Leg fatigue, Borg scale | 2 [1; 4] | 2 [1; 4] | 3 [1; 5] | 0.080 |
| Cardiopulmonary exercise test | ||||
| Wmax, watts | 57 (27) | 68 (27) | 43 (20) | <0.001 |
| Wmax, % predicted | 44 (21) | 52 (20) | 33 (17) | <0.001 |
| VO2peak, mL/min | 1016 (369) | 1185 (350) | 795 (261) | <0.001 |
| VO2peak, % predicted | 62 (21) | 71 (18) | 50 (19) | <0.001 |
| VO2peak, mL/min/kg | 13 (3) | 15 (3) | 10 (2) | <0.001 |
| VCO2peak, mL/min | 1092 (445) | 1289 (436) | 838 (309) | <0.001 |
| VEmax, % predicted | 110 (27) | 113 (28) | 107 (26) | 0.040 |
| MVV, L | 45 (20) | 54 (20) | 34 (14) | 0.001 |
| Ventilatory reserve, % | 9.5 (23.0) | 12.3 (21.3) | 5.9 (24.8) | 0.092 |
| Peak HR, bpm | 125 (18) | 126 (19) | 124 (17) | 0.525 |
| Oxygen pulse mL/beat | 8.1 (2.7) | 9.4 (2.5) | 6.5 (2.1) | <0.001 |
| Oxygen pulse, % predicted | 65 (27) | 73 (30) | 54 (17) | <0.001 |
| Final SpO2, % | 94 [91; 96] | 95 [93; 97] | 93 [90; 95] | <0.001 |
| Final Dyspnea, Borg scale | 5 [4; 6] | 4 [3; 6] | 7 [4; 8] | 0.003 |
| Final Leg fatigue, Borg scale | 5 [4; 6] | 5 [4; 6] | 5 [2; 8] | 0.961 |
Definition of abbreviation: CPET: cardiopulmonary exercise test; 6MWD: six-minute walk distance; VO2: oxygen consumption; Wmax: maximum work-load; VCO2: carbon dioxide output; VE: minute ventilation; MVV: maximal voluntary ventilation; HR: heart rate; SpO2: pulse oximetry oxygen saturation.
Values are expressed as mean (SD) when data is normally distributed or as median and interquartile range [P25–P75] when data distribution is skewed.
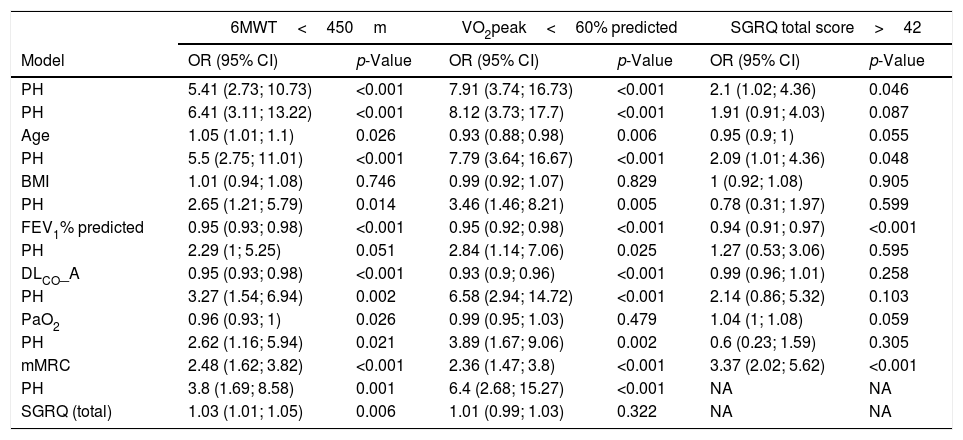
The logistic regression model showed that the presence of PH was associated with poor exercise capacity, adjusted by lung function, age, and BMI. In the analysis, the group of patients with a distance covered in the 6MWT below the median value (<450m) show an OR of 5.41. The OR in the adjusted models by age, BMI, FEV1%pred, and PaO2 were statistically significant. In the VO2peak analysis (<60%pred) the group with PH shows an OR of 7.91 (3.74; 16.73) (p<0.001). The OR in the adjusted models by age, BMI, FEV1%pred, and PaO2 were statistically significant. In the quality of life analysis (SGRQ score>42) the group with PH shows an OR of 2.1 (1.02; 4.36) (p=0.046). The OR in the adjusted models by age, BMI, and FEV1%pred were statistically significant (Table 3).
Logistic regression models for patients with lower maximal and submaximal exercise capacity and worse quality of life.a
| 6MWT<450m | VO2peak<60% predicted | SGRQ total score>42 | ||||
|---|---|---|---|---|---|---|
| Model | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| PH | 5.41 (2.73; 10.73) | <0.001 | 7.91 (3.74; 16.73) | <0.001 | 2.1 (1.02; 4.36) | 0.046 |
| PH | 6.41 (3.11; 13.22) | <0.001 | 8.12 (3.73; 17.7) | <0.001 | 1.91 (0.91; 4.03) | 0.087 |
| Age | 1.05 (1.01; 1.1) | 0.026 | 0.93 (0.88; 0.98) | 0.006 | 0.95 (0.9; 1) | 0.055 |
| PH | 5.5 (2.75; 11.01) | <0.001 | 7.79 (3.64; 16.67) | <0.001 | 2.09 (1.01; 4.36) | 0.048 |
| BMI | 1.01 (0.94; 1.08) | 0.746 | 0.99 (0.92; 1.07) | 0.829 | 1 (0.92; 1.08) | 0.905 |
| PH | 2.65 (1.21; 5.79) | 0.014 | 3.46 (1.46; 8.21) | 0.005 | 0.78 (0.31; 1.97) | 0.599 |
| FEV1% predicted | 0.95 (0.93; 0.98) | <0.001 | 0.95 (0.92; 0.98) | <0.001 | 0.94 (0.91; 0.97) | <0.001 |
| PH | 2.29 (1; 5.25) | 0.051 | 2.84 (1.14; 7.06) | 0.025 | 1.27 (0.53; 3.06) | 0.595 |
| DLCO_A | 0.95 (0.93; 0.98) | <0.001 | 0.93 (0.9; 0.96) | <0.001 | 0.99 (0.96; 1.01) | 0.258 |
| PH | 3.27 (1.54; 6.94) | 0.002 | 6.58 (2.94; 14.72) | <0.001 | 2.14 (0.86; 5.32) | 0.103 |
| PaO2 | 0.96 (0.93; 1) | 0.026 | 0.99 (0.95; 1.03) | 0.479 | 1.04 (1; 1.08) | 0.059 |
| PH | 2.62 (1.16; 5.94) | 0.021 | 3.89 (1.67; 9.06) | 0.002 | 0.6 (0.23; 1.59) | 0.305 |
| mMRC | 2.48 (1.62; 3.82) | <0.001 | 2.36 (1.47; 3.8) | <0.001 | 3.37 (2.02; 5.62) | <0.001 |
| PH | 3.8 (1.69; 8.58) | 0.001 | 6.4 (2.68; 15.27) | <0.001 | NA | NA |
| SGRQ (total) | 1.03 (1.01; 1.05) | 0.006 | 1.01 (0.99; 1.03) | 0.322 | NA | NA |
Analysis restricted to patients with values below the median. Data are shown in Odds Ratio (OR) and 95% confidence interval (95%CI).
Definition of abbreviation: PH: pulmonary hypertension; FEV1: force expiratory volume in the first second; DLCO: diffusion capacity of the lung for CO; mMRC: modified Medical Research Council scale; SGRQ: Sant George's Respiratory Questionnaire.
The main finding of this study is that the presence of PH was associated with impaired exercise capacity in COPD patients adjusted for age, BMI and airflow obstruction. Patients with increased PAP reached lower maximal work-load while cycling, presented lower VO2peak, covered less distance in the 6MWT, developed greater O2 desaturation during exercise, and had more dyspnea and worse quality of life. Although the ventilatory impairment and the reduced PaO2 are important factors limiting exercise in COPD patients, the multivariate analysis shows that PH per se has a detrimental effect on exercise capacity for a given degree of ventilatory impairment.
Although these results are expected, it is necessary to characterize pulmonary hemodynamics in patients with moderate to severe COPD and to analyze their response to exercise to choose better therapeutic strategies, especially for those who are candidates for pulmonary rehabilitation. In keeping with this, previous studies have shown that pulmonary rehabilitation improve exercise capacity in patients with COPD-PH but concomitant treatment with pulmonary vasodilators do not improve the results of the pulmonary rehabilitation program in exercise tolerance.27 Additionally the most recent Task Force on Pulmonary Vascular Diseases also recommended pulmonary rehabilitation in patients with COPD and moderate PH.16
Patients with COPD-PH showed a profile of exhausted circulatory reserve during exercise. The oxygen pulse, that provides an estimate of left ventricle stroke-volume changes during exercise,28 was significantly lower in the COPD-PH group (54% predicted) than in COPD patients without PH (73% predicted). These results are in line with the study by Boerrigter et al.17 who showed in their patients with severe PH an exhausted circulatory reserve at the end of the exercise while the breathing reserve was maintained.17
Exercise limitation in patients with COPD was initially associated as a direct consequence of airway obstruction, but this association has shown only a weak relation.29 The dynamic hyperinflation leads by the limitation of expiratory flows has been associated with functional weakness of the inspiratory muscles and with the restriction of thoracic expansion of normal tidal volume during exercise.30 On the other hand, changes in skeletal muscle are associated with exercise limitation.31 In the case of the presence of PH in patients with COPD, the literature describes the limited exercise capacity in patients with COPD and abnormal pulmonary artery pressure.15 Our group showed differences between lung function, but when correcting by this factor, it still appears this difference in exercise capacity, that can be explained by the increase of PAP.
We observed that the presence of PH decreased oxygen uptake and oxygen pulse, in addition to the ventilatory impairment typically observed in COPD patients. Based on the Fick principle, O2 pulse depends on both stroke volume and arteriovenous oxygen difference (Ca-vO2). For the cardiovascular nature of PH, these patients have demonstrated alterations in stroke volume.32 On the other hand, abnormalities on arterial blood gases have been described as playing a role in exercise intolerance.33 In patients with PH, hypoxemia accelerates the early occurrence of lactic acidosis increasing the ventilatory drive (by stimulation of the carotid bodies) contributing to an excessive increase in VE during exercise.34 This is another contributing factor for the reduction in exercise tolerance in our patients, reproduced at both exercise tests.
Cuttica et al., in a retrospective analysis of almost 5000 patients with advanced COPD, candidates for lung transplantation, reported that PH of any cause resulted in significant functional impairment measured by 6MWT and this result was associated with an increased risk of mortality.3 Likewise, Sims et al., in 362 patients with severe COPD who were evaluated for lung transplantation, demonstrated that higher values of mPAP were associated with impaired exercise tolerance measured by 6MWT, independently of the severity of the airflow obstruction.10 Our results are in line with these previous reports in a population of patients less severely impaired; we also found that the 6min walk distance (6MWD) in our group with COPD-PH was about 100m lower than in the group without PH (p<0.001). Contrasting with these findings, the 6MWD did not differ in other similar studies although evaluating a smaller sample of patients.11,12,14
To overcome these discrepancies, in our study we assessed comprehensively exercise tolerance by using maximal and submaximal exercise tests. We confirmed the impairment in exercise capacity in the COPD-PH group also in the CPET, the gold standard measurement for exercise capacity.
In our population, the VO2peak at the CPET was 33% lower in the group with PH, which is in line with previous studies.11,15 Although this finding could be due to lower FEV1 in the COPD-PH group, in the model adjusted by the FEV1 value, the probability of having a VO2peak<60% predicted was 3.5 higher in the COPD-PH group than in the non-PH COPD group.
Similarly, Vonbank et al., showed a significant decrease of 25% in VO2peak in COPD patients with PH, as compared with patients without PH.15 Thirapatarapong et al. also showed a reduction of 16% in VO2peak in COPD-PH patients.11 Of note, in both studies, there were no differences in the severity of airflow obstruction between groups. In contrast, other studies did not find a significant difference in VO2peak when comparing COPD-PH with non-PH COPD patients.12,35
In our study, the maximum workload achieved at the CPET was significantly lower in the COPD-PH group than in the non-PH group. This is in line with Thirapatarapong et al., who found significant difference in severe COPD patients (21(15)% predicted for COPD vs. 15(9)% predicted COPD-PH) and Skjorten et al., who studied 98 patients with a broad range of airway obstruction (72(31) watts for COPD vs 40(21) watts for COPD-PH).11,35 However, Holverda et al., Vonbank et al., Pynnaert et al., and Adir et al. did not find any difference.12–15 Furthermore, our results are more in line with the mechanistic explanation for PH because this loss of lung vasculature and distensibility is responsible for limiting exercise capacity.
In this type of patients, it is very critical the evaluation of the quality of life or multidimensional index like BODE index.36 Both measurements include the assessment of exercise tolerance and, we found a significant difference in both outcomes in the COPD-PH group (Table 1). Although these patients should be characterized with objective evaluations, we should not forget that patients are concerned about their symptoms and how these affect their quality of life, so they should be incorporated in the evaluation of the exercise capacity to explore the interaction between physical performance and quality of life.
Furthermore, patient's characteristics showed differences in pulmonary function, dyspnea and quality of life between COPD-PH and non-PH COPD group. These differences might explain, in part, the difference in maximum workload or VO2peak between the groups. However, we applied a logistic regression model with the objective to adjust important variables that influence during exercise, such as age, BMI, and very important in respiratory patients, FEV1%pred. These models were applied in submaximal (6MWD<450m) and maximal (VO2peak<60% pred) exercise tests, and in all these models, we found differences in exercise capacity between both groups. All these findings may suggest that the presence of PH plays an important role in limiting exercise capacity.
The characterization of pulmonary hemodynamics and exercise tolerance in patients with COPD-PH can contribute to improving their management, especially in the rehabilitation strategies. Recently, the European Respiratory Society (ERS) and the American College of Chest Physicians had developed guidelines for exercise in PH37,38; particularly, the ERS declares that the supervised exercise training may improve right ventricular function and pulmonary hemodynamics in patients with stable PH. Improved hemodynamics may contribute to an increase in exercise capacity and quality of life of patients. Unlike other chronic respiratory diseases, PH requires strict supervision of exercise in specialized centers, so the characterization of patients is essential in order to establish the most beneficial and least risky programs for each one.
This study has some limitations that ought to be disclosed. The first limitation of the study refers to the inclusion rates, which were different by the presence/absence of PH – this is, of course, important in assessing the risk of bias. Secondly, PH was assessed mostly through echocardiography. Although RHC is an invasive test not recommended for routine assessment in COPD patients, non-invasive measurement of PAP has shown good correlation with RHC39,40 and echocardiography is an acceptable screen for PH. Thirdly, in the context of COPD, pulmonary hypertension may involve the left heart (diastolic dysfunction possibly secondary to hyperinflation, associated systolic dysfunction) but, unfortunately, in our study, we could not exclude diastolic dysfunction in all patients (only in the 21% that had a RHC).
Finally, the decision to lower 10% the load in the constant-work test for PH patients precluded us to compare both groups statistically. Still, COPD-PH group reached shorter endurance time, and we can argue that with a 10% more of work-load the difference might have been significantly greater. We consider that endurance time is a very useful outcome to compare the results of an intervention such as a pulmonary rehabilitation program, especially in patients with PH because it reflects the resistance of the general musculature.
ConclusionsIn conclusion, PH in COPD patients impairs their exercise tolerance in maximal and submaximal tests and demonstrated a detrimental effect per se, irrespective of age, BMI, FEV1%pred and PaO2. Pulmonary hemodynamics should be considered to better understand the exercise intolerance in patients with moderate to severe COPD and associated PH to improve their management.
FundingThe study was supported by grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (PI17/1515), Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea. “Una manera de hacer Europa” Sociedad Española de Neumología y Cirugía Torácica (SEPAR) and Societat Catalana de Pneumologia (SOCAP). Elena Gimeno-Santos had personal funding from Instituto de Salud Carlos III, Sara Borrell contract (Acción Estratégica en Salud 2013–2016).
Conflict of interestThe authors declare no conflict of interest.
The authors would like to thank F. Burgos, C. Gistau, M. Palomo and X. Alsina (Pulmonary Medicine Department, Hospital Clinic, Barcelona, Spain) for their invaluable support and collaboration in the studies. Rodrigo Torres-Castro is a Ph.D. candidate in Methodology of Biomedical Research and Public Health Program, Universitat Autònoma de Barcelona, Spain.