Patent foramen ovale (PFO) is present in 10%–36% of the population. It is associated with minimal left-to-right shunting, although a transient right-to-left gradient may appear in early ventricular systole caused by a Valsalva maneuver – coughing, lifting heavy objects, or defecating. However, the clinical effect of right-to-left shunting through the PFO occurs only occasionally, and may present as a paradoxical embolism (e.g. stroke) or, more rarely, as platypnea-orthodeoxia syndrome (POS), with or without embolism. Cases of POS should be due not only to PFO, but also to the presence of an acquired abnormality. Thus, an anatomical defect in the form of interatrial communication should coexist with another functional defect that causes a change in direction of the blood flow when adopting a sitting or standing position.1,2 In this respect, it is important to note that POS has also been described in patients with pneumonectomy, intrapulmonary vascular malformations, right diaphragmatic paralysis, pericardial effusion, constrictive pericarditis, emphysema, cirrhosis, and amiodarone-induced lung disease.2,3
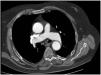
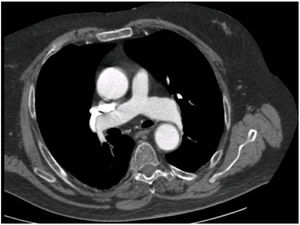
We report the case of a 76-year-old man with a history of obesity, prostate cancer, and vertebrobasilar and cerebellar stroke 5 years previously with no sequelae, unconfirmed suspected sleep apnea-hypopnea syndrome (SAHS) and ostium secundum-type interatrial communication. He presented with dyspnea and hypoxemia that had commenced a few months before admission, for which he was prescribed home oxygen, although the cause of the hypoxemia was never determined. At the time of admission, he had dyspnea at rest and presented disorientation and agitation, central cyanosis, tachypnea, inspiratory crackles in the right base and posterior plane, normal heart sounds with no signs of right overload and severe hypoxemia without hypercapnia. Chest radiograph showed no infiltrates, and pulmonary embolism was ruled out by computed tomography (CT) angiography, observing only some laminar atelectasia in dependent segments. Nevertheless, it was noted that the contrast density was higher in the aorta than in the pulmonary artery, despite the presence of residual contrast in the vena cava; it was also observed that the ascending aorta was dilated (Fig. 1). Arterial blood gas analysis with oxygen delivered by nasal cannula at a flow rate of 4Lpm in decubitus showed pH: 7.42; PaCO2: 38mmHg; PaO2: 56mmHg; HCO3: 25mEquiv./L; and SaO2: 89%. Transthoracic echocardiography (TTE) found good biventricular function with abnormal left ventricular relaxation, with no indirect signs of interatrial communication or pulmonary hypertension.
Application of noninvasive mechanical ventilation (NIMV) with FiO2 >60% did not significantly change the hypoxemia. However, when the patient was moved to a sitting position in the bed, he presented rapid hemoglobin desaturation. Agitated saline transesophageal echocardiography (TEE) showed the passage of a large number of bubbles from the right to the left atrium through a wide PFO, with filling of almost 80% of the left atrium in sitting position. We also observed a significant 25-mm displacement of the interatrial septum and a 45-mm dilation of the aortic root. Although it has been reported that performing TEE under NIMV support may be safer than conventional low oxygen therapy,4 hypoxemia was well tolerated and it was decided to perform the procedure with nasal cannula at 6Lpm. Finally, percutaneous closure of the PFO with an Occlutech® Figulla® device No. 30 (Occlutech GmbH, Jena, Germany) was performed, with clinical improvement being evident after the procedure. The patient was discharged without requiring oxygen therapy. Seven years after implantation of the occluder, correct placement of the device and absence of significant hypoxemia were confirmed.
In this case, we believe that dilation of the aortic root was the acquired factor. This finding has been published by different groups.1,2,5,6 It should also be mentioned that the presence of atelectasia worsened the hypoxemia, and although the SAHS was not confirmed, it has been reported that patients with SAHS and PFO can experience more desaturations in proportion to respiratory events than patients without PFO.7 Furthermore, we believe that the absence of echocardiographic signs of pulmonary hypertension increased the probability of successful closure.
In the case of a diagnosis of POS that cannot be explained by any other cause, even when TTE does not suggest shunting, a dynamic TEE should be performed in both the supine and sitting position, with or without a Valsalva maneuver. Another method for diagnosing right-to-left shunting is transcranial Doppler ultrasound, although TEE is preferred as it enables the site of the shunting to be confirmed and proper evaluation of the defect.2
In various published case series, percutaneous closure of the PFO has effectively resolved the POS.1,5,6,8–10 Monitoring the procedure by TEE is invaluable, as it allows us to confirm the optimal device size, check the absence of leaks once positioned—enabling it to be repositioned and/or removed if it does not fit properly—and to rule out procedural complications. Complications during and after implantation, though rare, can include: embolisms, infections, arrhythmias, device thrombosis, large persistent residual shunts, and traumatic fistulas between the aorta and left atrium, an event facilitated in cases of aortic aneurysm.6
Finally, it should be mentioned that in cases of PFO and pulmonary hypertension, closure is controversial because of the risk of right ventricular failure.11 Nevertheless, there are reports of patients with chronic obstructive pulmonary disease and PFO with right-to-left shunting studied by pulmonary catheterization, in whom the administration of both oxygen and inhaled nitric oxide produced a significant vasodilatory response together with improved oxygenation. In one such case, hypoxemia was resolved by percutaneous closure of the shunt, and in another, a significant improvement was noted following the administration of a phosphodiesterase-5 inhibitor.12
Please cite this article as: Navarro Esteva J, Ortega Trujillo JR. Tratamiento efectivo en ortodeoxia e hipoxemia grave. Arch Bronconeumol. 2020;56:333–334.