Research into the impact of hypoxia has focused on chronic continuous hypoxia (CCH), characteristic of COPD1 and chronic intermittent hypoxia (CIH), characteristic of sleep apnea–hypopnea syndrome (OSA).2,3 The co-existence of CCH and CIH results in a third type of hypoxia, “Overlap Syndrome”.4 The mechanisms of adaptation to these different types of hypoxia may be different, with changes on the cardiovascular system or the peripheral musculature, with a dose-response associated with hypoxia.5
We hypothesized that the three models of hypoxia behave differently in the expression of hypoxia-induced transcription factors (factor inducible hypoxia 1–2 and factor nuclear Kappa-B,NF-KB), as well as inflammatory biomarkers including tumor necrosis factor α (TNF-α), interleukin (IL)-8, IL-6, vascular endothelial growth factor (VEGF), vascular cell adhesion protein 1 (VCAM-1), and biomarkers of oxidative stress including superoxide dismutase (SOD) and catalase activities (CAT), both systemically and in the peripheral muscle. Our objective was to compare the mechanisms of adaptation in patients with three types of hypoxia, on the expression of transcription factors sensitive to hypoxia, the expression of systemic inflammation, oxidative stress level and antioxidant capacity, at the systemic and at the muscle levels.
This prospective, observational, cross-sectional study was conducted in male patients over 18 years, diagnosed with COPD, OSA or Overlap Syndrome, further a control group. All subjects underwent a clinical evaluation, spirometry with bronchodilator testing and a home respiratory polygraphy (Embletta Gold PG System).
The COPD patients had FEV1<80% in post-bronchodilator spirometry, a daytime arterial partial pressure of oxygen (PaO2)<70mmHg and an apnea–hypopnea index (AHI)<5. The OSA patients had FEV1>80%, a PaO2>80mmHg, an AHI>15 and had not received CPAP treatment previously. The overlap group had FEV1<80%, a PaO2<70mmHg and an AHI>15. The control group had a normal spirometry with an AHI<5.
Blood samples were collected by venipuncture and were immediately centrifuged, and plasma aliquots were stored at −80°C. Muscle biopsy samples were obtained from all patients (not in control group) from the vast lateral of the quadriceps muscle, frozen in liquid nitrogen and preserved at −80°C. For the analysis in plasma, we selected hypoxia-associated transcription factors, inflammatory, oxidative stress and antioxidants biomarkers. In the muscle biopsies we analyzed the gene expression of the inflammatory markers using reverse transcription quantitative polymerase chain reaction (RT-qPCR).
The study included 34 subjects: 4 with COPD, 12 with OSA, 13 with overlap and 5 controls.
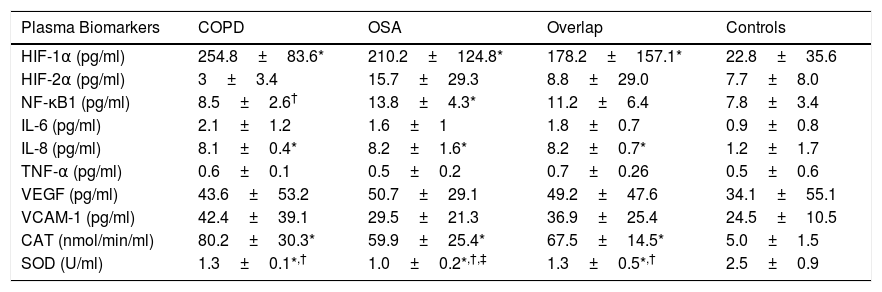
The plasma concentration of transcription factors, inflammation and oxidative stress markers presented significant differences in the three groups of patients compared to the controls (Table 1). In plasma, the NF-KB1 was higher and SOD was significantly decreased in the OSA group compared with COPD and Overlap Syndrome.
Plasma Concentration of Transcription Factors, Inflammation and Oxidative Stress Markers, and Muscle Gene Expression of Transcription Factors, Inflammation and Oxidative Stress Parameters.
| Plasma Biomarkers | COPD | OSA | Overlap | Controls |
|---|---|---|---|---|
| HIF-1α (pg/ml) | 254.8±83.6* | 210.2±124.8* | 178.2±157.1* | 22.8±35.6 |
| HIF-2α (pg/ml) | 3±3.4 | 15.7±29.3 | 8.8±29.0 | 7.7±8.0 |
| NF-κB1 (pg/ml) | 8.5±2.6† | 13.8±4.3* | 11.2±6.4 | 7.8±3.4 |
| IL-6 (pg/ml) | 2.1±1.2 | 1.6±1 | 1.8±0.7 | 0.9±0.8 |
| IL-8 (pg/ml) | 8.1±0.4* | 8.2±1.6* | 8.2±0.7* | 1.2±1.7 |
| TNF-α (pg/ml) | 0.6±0.1 | 0.5±0.2 | 0.7±0.26 | 0.5±0.6 |
| VEGF (pg/ml) | 43.6±53.2 | 50.7±29.1 | 49.2±47.6 | 34.1±55.1 |
| VCAM-1 (pg/ml) | 42.4±39.1 | 29.5±21.3 | 36.9±25.4 | 24.5±10.5 |
| CAT (nmol/min/ml) | 80.2±30.3* | 59.9±25.4* | 67.5±14.5* | 5.0±1.5 |
| SOD (U/ml) | 1.3±0.1*,† | 1.0±0.2*,†,‡ | 1.3±0.5*,† | 2.5±0.9 |
| Muscle biomarkers | COPD | OSA | Overlap |
|---|---|---|---|
| HIF-1α (pg/ml) | 0.052±0.078 | 0.05±0.034 | 0.058±0.05 |
| HIF-2α (pg/ml) | 0.13±0.157 | 0.17±0.09 | 2.33±6.72 |
| NF-κB1 (pg/ml) | 0.38±0.733 | 0.065±0.062 | 0.062±0.049 |
| IL-6 (pg/ml) | 0.007±0.009 | 0.007±0.008 | 0.006±0.006 |
| IL-8 (pg/ml) | 0.022±0.02 | 0.463±1.4 | 0.372±0.8 |
| TNF-α (pg/ml) | 0.03±0.06 | 0.002±0.003 | 0.005±0.009 |
| VEGF (pg/ml) | 0.165±0.31 | 0.126±0.08 | 0.218±0.43 |
| VCAM-1 (pg/ml) | 0.105±0.03† | 0.05±0.04 | 0.09±0.08 |
| CAT (nmol/min/ml) | 1.432±2.2 | 0.733±0.3 | 1.295±1.4 |
| SOD (U/ml) | 4.577±3.6† | 13.856±9.87 | 15.954±17.5 |
Abbreviations: COPD: chronic obstructive pulmonary disease; OSA: sleep apnea–hypopnea syndrome; HIF-1α: hypoxia inducible factor 1, subunit α; HIF-2α: hypoxia inducible factor 2, subunit α; NF-κB1: nuclear factor kappa-B 1; IL- 6: interleukin-6; IL- 8: interleukin-8; TNF-α: tumor necrosis factor alpha; VEGF: vascular endothelial growth factor; VCAM-1: vascular cell adhesion protein 1; CAT: catalase activity; SOD: superoxide dismutase activity.
As regards the muscle gene expression of transcription factors (Table 1), we found that in COPD patients the expression of VCAM-1 was higher and SOD was lower than in OSA and overlap groups.
In the OSA group we found a significant correlation (r=0.579; P=.04) between the levels of NF-KB1 obtained in plasma and in muscle, besides a significant correlation between the inflammatory and oxidative biomarkers in plasma and in muscle: plasma IL8-muscle SOD (r=0.69; P=.01); plasma endothelin-muscle CAT (r=0.61; P=.03); plasma ICAM-muscle IL8 (r=0.71; P=.009) and plasma ICAM-muscle CAT (r=0.80; P=.002).
In COPD group there was an increase in muscle expression of VCAM-1 compared to OSA, and a decreased in SOD compared with the other two groups.
The three groups of hypoxemic patients show an overexpression of hypoxia-sensitive transcription factors, primarily HIF-1α, compared to control subjects. In OSA there was an increase in plasma levels of NF-κB1 compared to COPD group. In situations of CIH, overexpression of NF-κB1 in plasma has been demonstrated in a cell culture model6 with a selective activation of inflammatory pathways, unlike CCH hypoxia of patients with COPD and overlap, where the activation of adaptive pathways predominates. Oxidative stress driven by CIH may initiate systemic inflammation in OSA,7 which inflammatory response in turn promoting the development of cardiovascular morbidities.8,9 We have found a decrease in the plasma of SOD in OSA patients. In situations with a chronically oxidative stress due to overexpression of NF-κB1, there may be a decrease in SOD by a saturation of the antioxidant systems.
We found no differences between the three patient groups in the expression of transcription factors in peripheral musculature. However, in COPD there is a higher level of VCAM and lower SOD than in OSA, reflecting a more intense inflammatory state at the muscle, besides a lower ability to nullify the deleterious effect of free radicals. These findings match with the findings of other authors10 and agree with that muscle dysfunction in COPD is clinically important. In patients with CCH, inflammatory and oxidative processes in plasma and in muscle fiber seem to be independent. In OSA, there is a correlation between the levels of the NF-KB in plasma and in muscle fiber, as well as between parameters of inflammatory and antioxidant status in plasma and peripheral muscle, suggesting that inflammation and oxidative stress at the systemic levels and in the muscle are interrelated processes.
In summary, the three groups of hypoxemic patients show an overexpression of hypoxia-sensitive transcription factors, with a higher expression of NF-κB in OSA. In plasma, an increase in oxidative stress is evident in the three groups, but the deficit in antioxidant substances is greater in OSA, which may be due to the overexpression of NF-κB. At peripheral muscle, there were no differences in inflammatory parameters, unlike the response to oxidative stress, with an increase in the production of free radicals and a decrease in antioxidant substances in COPD patients versus OSA patients. Inflammation and oxidative stress in plasma and muscle in COPD and COPD-OSA seem to be independent phenomena. In OSA, inflammatory and oxidative stress levels in muscle fiber are related to systemic levels.
Funding SourceGrants for projects of the Association of Pulmonologists of the South. Neumosur. Project: Expression of transcription factors sensitive to hypoxia, inflammatory response and oxidative stress at the systemic and muscular level in three clinical models of hypoxemia.
Research grants 2012 SEPAR. Code: 051|2012. Expression of transcription factors sensitive to hypoxia, the inflammatory response, oxidative stress and peripheral muscle involvement in three clinical models of hypoxemia.
Conflicts of InterestNone declared.