The diaphragm is the main respiratory muscle, and is formed by two muscles with dual innervation, joined by a central tendon. When it is contracted, the caudal movement increases the volume of the rib cage, generating the negative pressure necessary for inspiratory flow. Diaphragmatic dysfunction is caused by anatomical, neuromuscular, or biomechanical problems that affect this contraction.1
One of the techniques for assessing the standard pattern of diaphragmatic dysfunction is transdiaphragmatic pressure. However, it is invasive, incapable of discriminating between uni- or bilateral involvement, and is rarely used in clinical practice. Other techniques, such as static maximal inspiratory mouth pressure (PImax) or sniff nasal inspiratory pressure (SNIP) and vital capacity in supine, are noninvasive and simple, but they lack sensitivity and cannot discriminate between uni- or bilateral involvement. Imaging techniques include the more conventional radioscopy (low sensitivity if the involvement is bilateral), as well as highly sophisticated tools, such as cine-mode computed tomography or cine-mode magnetic resonance imaging, that show 3-dimensional movement and muscle content. However, these modalities are expensive and complex and, as such, impractical for routine diaphragmatic evaluation.2
The recent development of diaphragmatic ultrasound has revolutionized diaphragm evaluation, as it can be used to assess bilateral diaphragmatic morphology and function in real time, permitting follow-up without exposure to radiation. It is, moreover, affordable and ubiquitous.
Several methods have been proposed to evaluate diaphragmatic movement since the first description published by Haber in 1975.3 Nowadays, two main approaches are used: the movement of the dome, and muscle thickening.4,5
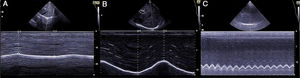
The ability of the diaphragm to generate volume changes in the rib cage is evaluated by measuring its movement. When the subcostal approach is used, a low-frequency convex transducer is placed at the level of the mid-clavicular line, seeking the posterior third of the diaphragmatic dome in the right side, through the liver window. In the left side, the splenic window is used, but the dome is more difficult to locate, especially in the presence of interposing abdominal viscera.6 An alternative is the lateral approach, placing the probe in a perpendicular position in the intercostal spaces below the level of the mid-axillary line. After the dome is located in 2D mode, anatomical M mode ultrasonography is used to help identify the portion of the dome with greatest mobility, which is then measured in a sagittal slice. Respiratory movement is measured at rest (tidal volume), in deep inspiration and expiration (vital capacity), and in sniff maneuvers (Fig. 1). The force of the movement can also be measured (movement over time, visualized as the slope of the movement curve). In some situations in which the diaphragm is not properly differentiated, the movement of abdominal viscera can be measured (vena cava in the right side, or the spleen on the left), with acceptable results. This is known as the “indirect method”.7
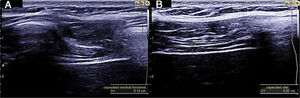
Measuring diaphragmatic thickness is another method currently used to determine diaphragmatic muscle mass and assess the thickening fraction (TF). For this measurement, a high-frequency linear transducer is used, placed between the last intercostal spaces. This locates the zone of apposition, where the diaphragm is in direct contact with the chest wall. The diaphragm is identified as a hypoechoic strip flanked by two hyperechoic lines, which correspond to the pleura and peritoneum. The TF is the ratio between diaphragm thickness at rest (functional residual capacity) and in maximum inspiration (vital capacity) (Figs. 1 and 2).
Various authors have proposed normal values for both measurements.6,8
These measurements have a series of important practical applications. One of the most widely studied is the prediction of successful weaning from mechanical ventilation in critically ill patients: TF>30% is associated with greater success in weaning, while TF<20% predicts prolonged weaning, and this parameter helps evaluate mechanical ventilation-induced diaphragmatic damage.4
Ultrasound in the assessment of the neuromuscular patient has shown promising results in diseases with special predilection for the diaphragm, such as glycogen storage disease or mitochondrial myopathies. It is useful in patients with bulbar involvement that prevents the performance of traditional functional respiratory tests. In particular, it has proven to be a predictor of hypoventilation in patients with ALS,2,9 although this claim has been disputed by other authors.10
In COPD patients, diaphragmatic ultrasound has been proposed to evaluate the effect of rehabilitation on the diaphragm has been proposed,11 and it is described as a predictor of the success of non-invasive mechanical ventilation in patients with severe exacerbations.12
It is also useful in the detection and follow-up of postoperative diaphragmatic dysfunction after surgery that might involve phrenic nerve injury, such as cardiac or thoracic interventions. The integrity of the phrenic nerve can be evaluated by stimulating its cervical motor pathway and observing the response in diaphragmatic displacement and thickening.
In other applications, such as titration of the stimulation thresholds of a phrenic nerve pacemaker, diaphragmatic ultrasound could replace more invasive techniques, such as the use of transesophageal diaphragmatic pressure.13
In our opinion, however, some obstacles need to be overcome before diaphragmatic ultrasound is fully developed. These include, among others, the absence of standard normal values based on large populations (similar to the predictive equations used in spirometry) and the variability in measurements depending on the patient’s position or location of the transducer. Other issues, such as the need to establish a prior period of spontaneous ventilation before evaluating TF in patients receiving mechanical ventilation, need to be taken into account when using this technique. Even when disconnecting to spontaneous ventilation, muscle fatigue may take a few minutes to manifest before it can be evaluated.14
Diaphragmatic ultrasound is an interesting development in modern respiratory medicine that will soon be added to the diagnostic arsenal of the pulmonologist. The ease with which operators gain experience in its use, along with its rapid learning curve, will also facilitate its speedy implementation.15 These factors, coupled with the ubiquitous availability of ultrasound equipment, use at the bedside, and foreseeable technological improvements will transform this technique into an additional tool in the functional assessment of the respiratory muscle. In order to generalize its use even further, standard normal values must be determined in large cohorts, measurement techniques must be protocolized, and its sensitivity/specificity in the different diseases must be more precisely defined.
Conflict of interestsJavier Sayas has received honoraria for teaching tasks related to mechanical ventilation from Chiesi, ResMed, Philips, and Mundipharma. He is the principal investigator of a project that is partially funded by Menarini, and an associate researcher in a project that includes the use of diaphragmatic ultrasound funded by Sanofi.
Ana Hernandez-Voth is the principal investigator of a project funded by Sanofi, and has received honoraria for teaching activities from Chiesi.
Victoria Villena has received honoraria for teaching activities from Menarini.
The authors would like to thank Dr. Borja de la Quintana Gordon for his dedication to education and for his collaboration.
Please cite this article as: Catalán JS, Hernández-Voth A, Garrido MVV. Ecografía diafragmática: una herramienta de novedosa a rutinaria. Arch Bronconeumol. 2020;56:201–203.