Despite of the demonstrated benefits of an early diagnosis and treatment on disease progression,1 today the diagnosis of alpha1-antitrypsin deficiency (AATD) remains a challenge in daily clinical practice. Different barriers have been identified during the past years including a low suspicion level mainly based on a misconception of the disease among clinicians, who only suspect the disease in a selected and infrequent group of patients.2 As a consequence, the degree of alpha1-antitrypsin (AAT) serum determination is frequently insufficient.3,4 Accordingly, different initiatives have been developed to improve diagnosis over the past years. Based on lateral-flow paper-based technologies,5 the Alphakit Quickscreen (Grifols, Barcelona, Spain) has been marketed in Europe for the identification of the Z protein (PiZ) in serum. Therefore, the test allows the identification of the Z allele homozygous, heterozygous and carries
The opportunities for the diagnosis of AATD of such a test are clear, since the on-site rapid and accurate identification of PiZ carriers may help identify AATD cases at an early stage with implications for management and family screening. However, the diagnostic accuracy of this test has been scarcely studied. In the one real world evaluation of its performance available,6 the evaluation of the test's ability to detect the PiZ protein showed a specificity of 97.8%, sensitivity of 73.8%, negative predictive value of 98.9%, and positive predictive value of 58.5%. Of note, after exploring the test-performance with different prevalence pre-test probability, the authors found lower negative predictive values in a population with a very high pre-test probability. Therefore, the authors concluded that the device could be used as an appropriate tool to exclude AATD in primary care and in the overall COPD population, except in patients with a high a priori-probability of AATD. Additionally, an added finding was that all false negatives (n=11) were heterozygote Pi*MZ samples, with a correct identification of ZZ and SZ genotypes. In this context, an unexplored population of special interest is that with a low a priori pre-test probability of severe AATD defined as those with AAT serum concentration>50 mg/dl which are associated with increased risk for COPD7. The identification of the Z allele in this population may help identifying Z carriers and advance in the early identification of treatable cases.
We performed a prospective, single-center, observational, cross-sectional, real-world analysis on the performance of Alphakit Quickscreen. Following the Spanish recommendations,8 all patients referred to our COPD-dedicated outpatient clinic from January 2016 to July 2018 for a diagnosis of COPD had a serum AAT determination by nephelometry and those between 50 and 120mg/dL, had an Alphakit Quickscreen determination done. The test was performed complying with all the requirements of the manufacturer in the conservation and performance of the test. The study was completed by allele-specific genotyping. The diagnostic profile including the sensitivity, specificity, and the positive and negative predicted values for the identification of the Z allele was calculated together with the 95% confidence interval (95%CI). Data are expressed as absolute (relative) frequencies and mean (standard deviation) according to the variables’ nature.
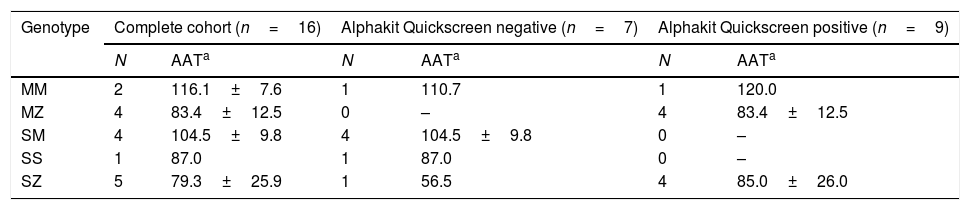
The sample was composed of 16 subjects: 11 (68.8%) males, 58.4 (15.3) years of age, 6 (37.5%) current smokers, tobacco history 52.7 (31.7) pack-year, body mass index 27.7 (6.8) kg/m2, of which 12 (75.0%) fulfilled COPD diagnostic criteria with a post-bronchodilator FEV1 of 64.3 (18.0) %. Serum AAT concentrations varied from 56.5 to 120.0mg/dL, with a mean value of 91.7 (20.7) mg/dL. The distribution of the Z alleles according to the Alphakit Quickscreen result is presented in Table 1. The prevalence of the Z allele in this highly selected low-risk population of cases was 56.2% (95%CI 31.9–80.5). The diagnostic profile of the test resulted in a specificity of 85.71 (95%IC 68.5–100), sensitivity 88.9 (95%IC 73.5–100), negative predictive value 85.7 (95%CI 68.5–100), and positive predictive value 88.9 (95%CI 73.5–100). We detected one false negative (a SZ case with AAT serum concentration of 56.5mg/dL) and one false positive (a MM case with AAT serum concentration of 120mg/dL).
Results of the Alphakit Quickscreen test and the genotyping.
The Alphkit Quickscreen represents an attractive diagnostic method since it explores the presence of the Z protein using a non-invasive method and is freely distributed in the country by Grifols (Grifols, Barcelona, Spain). Our data show the diagnostic profile of Alphakit Quickscreen device in an a priori low probability of AATD cohort and additionally describing one case of a false negative in a SZ patient and a false positive in a MM case. The main strength of our approach is the exploration of a specific subgroup of patients in which the test may be useful. The limitation is the small sample size which is intrinsically associated with the population selection in a rare disease like AATD. Therefore, these findings should be confirmed in an independent cohort that includes these types of patients.
The evaluation of the diagnostic profile of new diagnostic techniques is a necessary step before wide clinical implementation. The one previous real-world available study evaluated 1019 samples in test-naïve COPD patients from 9 centers in Spain and 10 centers in Germany. Patients included were ≥30 years of age and with a confirmed diagnosis of COPD, with no exclusion criteria regarding the AAT serum levels. Our study continues the evaluation of the Alphakit Quickscreen in a specific population which may be of interest for the identification of Z-allele carriers. Our area has a lower prevalence of PI*Z as compared to the rest of the country.9 As a quick, easy, point-of-care test, this may have implications in the use of this test in future diagnostic algorithms. Although this device allows for rapid analysis at the patient's point of care, its actual position within a diagnostic algorithm as a screening tool needs to be assessed.
Another interesting finding is the identification of one case of SZ which was a false negative, as compared to the previous analysis in which all false negatives were MZ.6 False negatives in a lateral-flow analysis may be related to preservation, technical performance or correct interpretation, rather than the specific genotype. Therefore, it is expected that false negatives may affect different Z-allele carriers irrespective of the final genotype, as the study of the test expands. Moreover, it would be interesting to confirm the genotype in all false negative and false positive cases by complete sequencing of coding exons of AAT gene.
In summary, the evaluation of the performance of Alphakit Quickscreen in an a priori low risk of AATD population shows a diagnostic profile that allows it to be considered as a potential step in the diagnostic algorithm of AATD diagnosis. Additionally, this device represents a great opportunity to increase the awareness of AATD.
Conflicts of interestJLLC has received during the last 3 years personal fees or non-financial support from Grifols and CSL Behring. The rest of the authors declare no conflicts of interest.
The authors would like to thank Grifols for providing the Alphakit Quickscreen for the present study.