To evaluate the usefulness of pleural fluid adenosine deaminase (ADA) for diagnosing tuberculous pleural effusions in the Spanish population, according to laboratory technique and cut-off point, and to compare the results with other populations.
MethodsMeta-analysis of diagnostic studies on pleural fluid ADA in the Spanish population, extracted from the PubMed and Embase databases from inception until July 2017, with no language restrictions. The overall diagnostic accuracy of ADA and that of each of the measurement techniques (Giusti, manual and automated kinetic methods) and selected cut-offs were analyzed. The QUADAS-2 tool was used to evaluate the quality of studies. A bivariate random effects model was used. Results were compared with those obtained from previous meta-analyses in non-Spanish populations.
ResultsSixteen studies with a total of 4147 patients, 1172 of whom had tuberculous pleural effusions, were included. ADA had 93% sensitivity, 92% specificity, positive likelihood ratio of 12, negative likelihood ratio of 0.08, and an area-under-the-curve of 0.968 for identifying tuberculosis. There were no differences in diagnostic accuracy between the techniques used for ADA measurement or the selected cut-offs. In 73 studies from non-Spanish populations a trend toward lower ADA sensitivity (88%, 95% CI: 86%–90%) and specificity (88%, 95% CI: 86%–90%) was noted, but differences did not reach statistical significance.
ConclusionsPleural fluid ADA in the Spanish population shows good diagnostic accuracy (regardless of the measurement technique or cut-off), similar to that reported in non-Spanish populations.
Determinar la utilidad de la adenosina desaminasa (ADA) pleural para diagnosticar derrame pleural tuberculoso en población española, según la técnica de medición y punto de corte utilizados, y compararla con la descrita para otras poblaciones.
MétodosMetaanálisis de estudios diagnósticos sobre ADA pleural en población española, extraídos de PubMed y Embase desde sus comienzos hasta julio de 2017, sin restricciones de lenguaje. Se analizó la eficacia diagnóstica global de la ADA, según sus técnicas de medición (Giusti, métodos cinéticos manuales y métodos cinéticos automatizados) y el punto de corte seleccionado. La herramienta QUADAS-2 evaluó la calidad de los estudios. Se utilizó un método bivariante de efectos aleatorios. Se compararon los resultados con los descritos en metaanálisis previos sobre población no española.
ResultadosSe incluyeron 16 estudios, con 4.147 pacientes, de los que 1.172 tenían derrame pleural tuberculoso. La ADA tuvo una sensibilidad del 93%, especificidad del 92%, likelihood ratio positiva de 12, likelihood ratio negativa de 0,08, y área bajo la curva de 0,968 para identificar tuberculosis. No hubo diferencias de eficacia diagnóstica entre las técnicas de medición de ADA o el punto de corte escogido. En 73 estudios de población no española se observó una tendencia hacia una menor sensibilidad (88%, IC95%: 86-90%) y especificidad (88%, IC95% 86-90%) de la ADA, pero las diferencias no alcanzaron significación estadística.
ConclusionesLa ADA pleural en población española tiene una buena precisión diagnóstica (independientemente de la técnica de medición o punto de corte empleados), similar a la reportada en población no española.
In 2016, 10.4 million new cases of tuberculosis (TB) occurred worldwide.1 In that year, the incidence of TB in Spain was 12 cases per 100000 inhabitants.1 In 2014, tuberculous pleural effusion (TPE) was the second most common form of extrapulmonary TB (18.4%) in Spain, after lymph node involvement (23.6%).2 In a Spanish series, TB was the fourth most common cause (9%) of 3077 pleural effusions submitted to thoracentesis, after cancer (27%), heart failure (21%), and pneumonia (19%).3
A definitive diagnosis of TPE requires tuberculous bacilli to be identified in specimens of sputum, pleural fluid (PF), or pleural biopsy. However, the yield of these microbiological studies is low, particularly when only solid culture media are used. Moreover, it takes several weeks to obtain precise results.4 Pleural biopsy, which reveals granulomas in 75% of cases, provides an earlier diagnosis, but is an invasive technique with inherent risks.5
Adenosine deaminase (ADA) is the most widely used biomarker for the diagnosis of TPE. In many hospitals, the determination of ADA in PE has replaced pleural biopsy for diagnostic purposes.4 The diagnosis of pleural TB in this way is usually accepted, and empirical TB treatment is started if the patient has a clinical picture of low-grade fever, respiratory symptoms, unilateral pleural effusion that corresponds to a lymphocytic exudate with cytological studies negative for malignancy and ADA≥35–40U/l (a diagnostic cut-off point that might be lower in older patients).4 To date, 6 meta-analyses have been published on the usefulness of this enzyme for diagnosing TPE, although none of the populations has been exclusively Spanish.6–11 These studies have some limitations. Firstly, none evaluates the effect of the different techniques for determining ADA or the selection of dichotomous cut-off points on the diagnostic efficacy of this approach. Secondly, most of these meta-analyses combined populations from different geographical areas with varying TB prevalences.6,7,9,10 The positive predictive value of pleural ADA is known to fall proportionally to the prevalence of TB, in such a way that in an area with a low prevalence of the disease, a raised pleural ADA value is more likely to be a false positive.4
The objectives of this study that differ from studies published to date are: (1) to evaluate the usefulness of pleural ADA in the diagnosis of TPE in a Spanish population, (2) to evaluate if the technique used to determine ADA or the selected cut-off point affects the diagnostic efficacy, and (3) to compare these results with of non-Spanish populations from already published meta-analyses.
Materials and MethodsSearch Strategy and Selection of StudiesA systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.12 The PubMed/MEDLINE and EMBASE electronic databases were consulted from their inception until July 2017. The search strategy used the following terms: (“pleural tuberculosis” OR “tuberculous pleuritis” OR “tuberculous pleurisy” OR “pleural”) AND (“adenosine deaminase” OR “ADA”). All the references of the selected articles were also reviewed. Two investigators (R.M.P. and S.B.) evaluated the studies independently and any discrepancies were resolved by mutual agreement.
Inclusion and Exclusion CriteriaStudies carried out in the Spanish population were included with no language restrictions, but had to meet 2 requirements: (1) the diagnosis of TPE was confirmed by pleural biopsy or microbiological culture of Mycobacterium tuberculosis in sputum, PF or pleural biopsy; and (2) sufficient data were available to construct a 2×2 contingency table that could be used to calculate diagnostic efficacy. Studies with duplicated or overlapping cases were excluded; if this was the case, the article with the greater sample size was selected.
Data ExtractionThe following characteristics were collected from the selected articles: authors, date of publication, study location, study design, number of patients and demographic data, methods for diagnosing TPE, etiology of non-tuberculous effusions, technique used to detect ADA in PF (Giusti, manual kinetic methods or automated kinetic methods), and the data needed to build a 2×2 table.
Evaluation of Study QualityTwo investigators (R.M.P. and S.B.) independently assessed the quality of the studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.13 This consists of 4 domains: (1) patient selection, (2) index test, (3) reference standard, and (4) flow and timing. Each domain is evaluated in terms of the risk of bias (low, high or uncertain), and the first 3 also for applicability.
Comparison With International StudiesAll the meta-analyses published on the diagnostic accuracy of pleural ADA in TB were identified,6–11 and studies performed in non-Spanish populations were selected. The complete text of these studies was read, and finally only those in which the TPE diagnosis had been confirmed by pleural biopsy or microbiological culture from a biological sample were taken into consideration. The same data as for the Spanish population, listed above, were extracted from each of the selected articles.
Statistical AnalysisContinuous variables were expressed as mean or median, and categorical variables as frequencies and percentages. Agreement between observers for the QUADAS-2 tool was determined using the non-weighted Cohen's kappa statistic; the result was considered good if it was 0.6 and excellent if higher than 0.8. Sensitivity, specificity, positive and negative likelihood ratio (LR), and diagnostic odds ratio (DOR) of pleural ADA, with corresponding 95% confidence intervals (95% CI), were calculated from a 2×2 table, using bivariate models of random effects. For the calculation of the LR, a correction of 0.5 was applied to all cells of the table if any of them contained the value 0. Due to the negative correlation between sensitivity and specificity, these parameters were analyzed simultaneously using a meta-regression of random effects, according to the sensitivity data and the rate of false positives obtained from the studies included.14 The summary ROC curve was estimated from this meta-regression. The meta-regression was adjusted for the techniques used to diagnose ADA in PF (Giusti, manual or automated kinetic method), and, if there were significant differences, the analysis was stratified for each one of the techniques separately. The same analytical process was followed adjusting for the ADA cut-off point used for the diagnosis of TB. Publication bias was estimated using a funnel plot for the measurements of positive and negative LR. Heterogeneity between studies was quantified using Higgins’ I2 statistic, estimated from the univariate meta-analysis based on the method of DerSimonian and Laird15 for 3 measures of the effect (positive LR, negative LR, and DOR). The level of statistical significance was set a 0.05. Calculations were performed using the R program (R-project; http://cran.r-project.org/web/packages/mada/index.html).
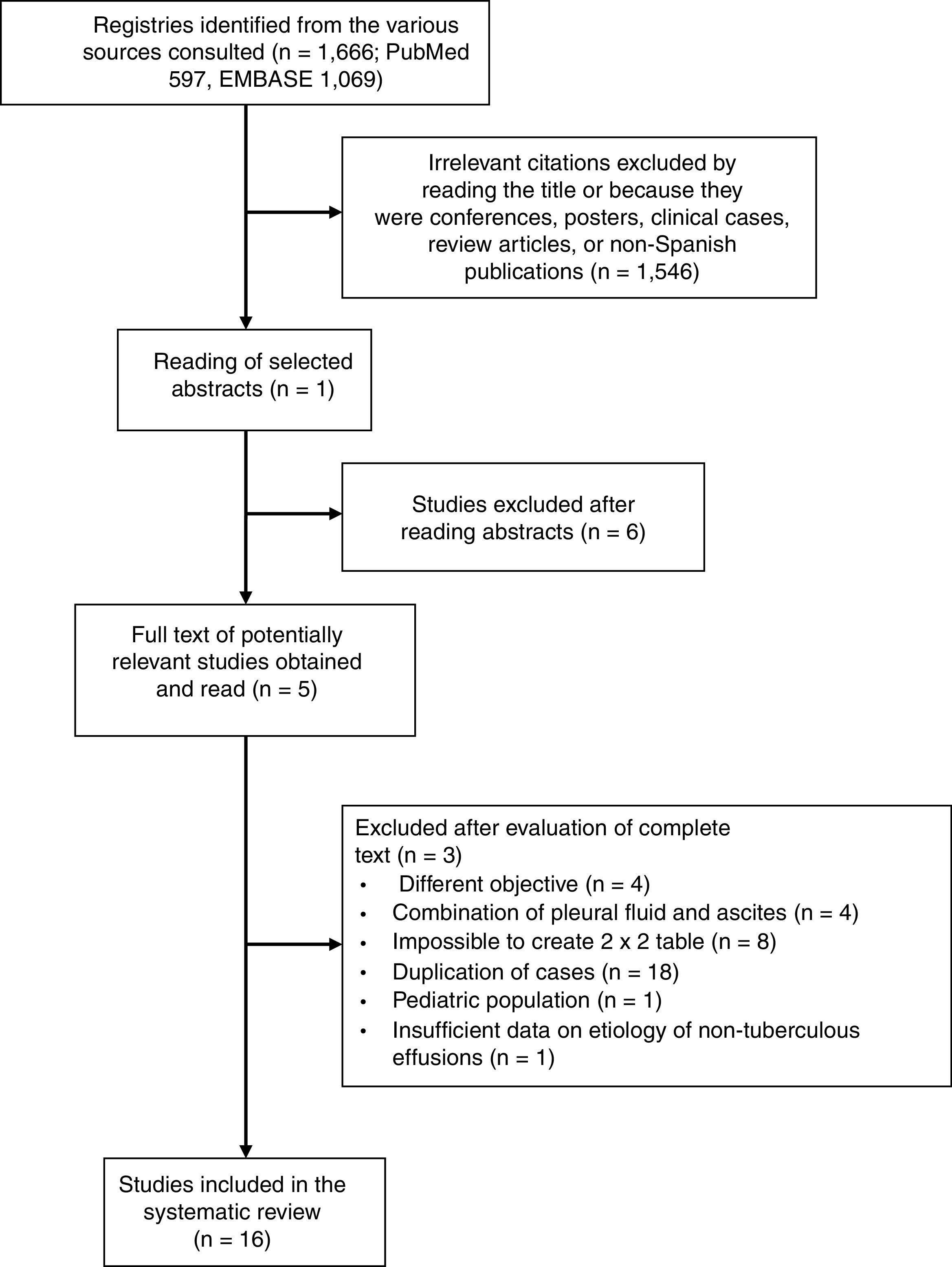
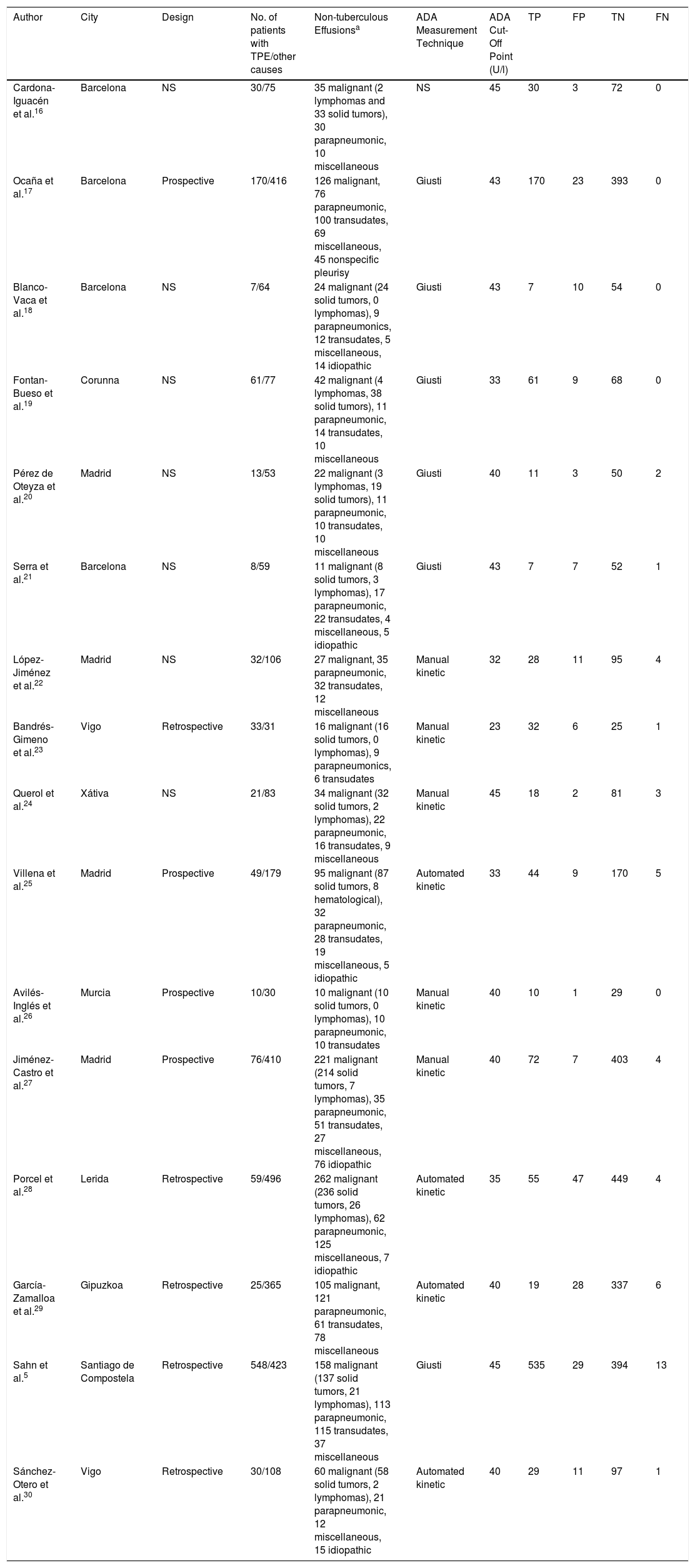
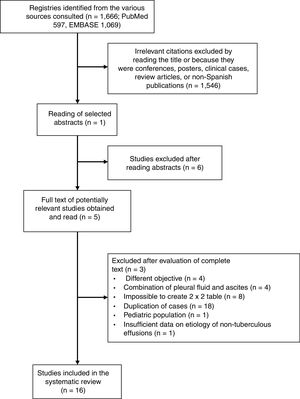
ResultsMeta-analyses in the Spanish PopulationIn total, 1666 articles were identified, and the complete text of 52 potentially relevant papers was read. Only 16 of these met the inclusion criteria5,16–30 (Fig. 1 and Table A.1). The selected studies comprised a total of 1172 patients with TPE and 2975 with effusion due to other causes, the most notable being malignant effusions (1248, 42%; of which 78 were lymphomas) and parapneumonic effusions (539, 18%; of which 74 were empyemas). Clinical characteristics of the study population are shown in Table 1. Several different techniques used to quantify ADA: 6 studies used the Giusti method,5,17–21 5 used manual kinetic techniques,22–24,26,27 and 4 used automated kinetic techniques.25,28–30 The ADA cut-off point for the diagnosis of TPE ranged between 2323 and 45U/l,5,16,24 although the most widely used was 40U/l.20,26,27,29,30 Age and sex of patients were not specified in 817,18,21–23,25–27 and 9 studies,17,21–28 respectively; and race was not specified in any.5,16–30 The QUADAS-2 tool did not detect a high probability of bias in any of its domains, although the patient selection domain was considered “uncertain” in 8 studies.16,18–24 Interobserver concordance for the evaluation of the QUADAS-2 domains was 0.9 (95% CI: 0.78–1).
Characteristics of Spanish Studies That Evaluated the Diagnostic Yield of Adenosine Deaminase in Pleural Fluid.
| Author | City | Design | No. of patients with TPE/other causes | Non-tuberculous Effusionsa | ADA Measurement Technique | ADA Cut-Off Point (U/l) | TP | FP | TN | FN |
|---|---|---|---|---|---|---|---|---|---|---|
| Cardona-Iguacén et al.16 | Barcelona | NS | 30/75 | 35 malignant (2 lymphomas and 33 solid tumors), 30 parapneumonic, 10 miscellaneous | NS | 45 | 30 | 3 | 72 | 0 |
| Ocaña et al.17 | Barcelona | Prospective | 170/416 | 126 malignant, 76 parapneumonic, 100 transudates, 69 miscellaneous, 45 nonspecific pleurisy | Giusti | 43 | 170 | 23 | 393 | 0 |
| Blanco-Vaca et al.18 | Barcelona | NS | 7/64 | 24 malignant (24 solid tumors, 0 lymphomas), 9 parapneumonics, 12 transudates, 5 miscellaneous, 14 idiopathic | Giusti | 43 | 7 | 10 | 54 | 0 |
| Fontan-Bueso et al.19 | Corunna | NS | 61/77 | 42 malignant (4 lymphomas, 38 solid tumors), 11 parapneumonic, 14 transudates, 10 miscellaneous | Giusti | 33 | 61 | 9 | 68 | 0 |
| Pérez de Oteyza et al.20 | Madrid | NS | 13/53 | 22 malignant (3 lymphomas, 19 solid tumors), 11 parapneumonic, 10 transudates, 10 miscellaneous | Giusti | 40 | 11 | 3 | 50 | 2 |
| Serra et al.21 | Barcelona | NS | 8/59 | 11 malignant (8 solid tumors, 3 lymphomas), 17 parapneumonic, 22 transudates, 4 miscellaneous, 5 idiopathic | Giusti | 43 | 7 | 7 | 52 | 1 |
| López-Jiménez et al.22 | Madrid | NS | 32/106 | 27 malignant, 35 parapneumonic, 32 transudates, 12 miscellaneous | Manual kinetic | 32 | 28 | 11 | 95 | 4 |
| Bandrés-Gimeno et al.23 | Vigo | Retrospective | 33/31 | 16 malignant (16 solid tumors, 0 lymphomas), 9 parapneumonics, 6 transudates | Manual kinetic | 23 | 32 | 6 | 25 | 1 |
| Querol et al.24 | Xátiva | NS | 21/83 | 34 malignant (32 solid tumors, 2 lymphomas), 22 parapneumonic, 16 transudates, 9 miscellaneous | Manual kinetic | 45 | 18 | 2 | 81 | 3 |
| Villena et al.25 | Madrid | Prospective | 49/179 | 95 malignant (87 solid tumors, 8 hematological), 32 parapneumonic, 28 transudates, 19 miscellaneous, 5 idiopathic | Automated kinetic | 33 | 44 | 9 | 170 | 5 |
| Avilés-Inglés et al.26 | Murcia | Prospective | 10/30 | 10 malignant (10 solid tumors, 0 lymphomas), 10 parapneumonic, 10 transudates | Manual kinetic | 40 | 10 | 1 | 29 | 0 |
| Jiménez-Castro et al.27 | Madrid | Prospective | 76/410 | 221 malignant (214 solid tumors, 7 lymphomas), 35 parapneumonic, 51 transudates, 27 miscellaneous, 76 idiopathic | Manual kinetic | 40 | 72 | 7 | 403 | 4 |
| Porcel et al.28 | Lerida | Retrospective | 59/496 | 262 malignant (236 solid tumors, 26 lymphomas), 62 parapneumonic, 125 miscellaneous, 7 idiopathic | Automated kinetic | 35 | 55 | 47 | 449 | 4 |
| García-Zamalloa et al.29 | Gipuzkoa | Retrospective | 25/365 | 105 malignant, 121 parapneumonic, 61 transudates, 78 miscellaneous | Automated kinetic | 40 | 19 | 28 | 337 | 6 |
| Sahn et al.5 | Santiago de Compostela | Retrospective | 548/423 | 158 malignant (137 solid tumors, 21 lymphomas), 113 parapneumonic, 115 transudates, 37 miscellaneous | Giusti | 45 | 535 | 29 | 394 | 13 |
| Sánchez-Otero et al.30 | Vigo | Retrospective | 30/108 | 60 malignant (58 solid tumors, 2 lymphomas), 21 parapneumonic, 12 miscellaneous, 15 idiopathic | Automated kinetic | 40 | 29 | 11 | 97 | 1 |
ADA, adenosine deaminase; FN, false negative; FP, false positive; NS, not specified; TN, true negative; TP, true positive; TPE, tuberculous pleural effusion.
Solid tumors include malignant tumors of the lung, breast, ovary, gastrointestinal tract, urinary tract, genitals, unknown origin and mesothelioma. Miscellaneous includes pleural effusions caused by pulmonary thromboembolism, Dressler's syndrome, surgical intervention, viruses, pericardial disease, hydatid cyst, trauma, pancreatitis, chylothorax, rheumatoid arthritis, sarcoidosis, porphyria, vasculitis, and transplantation.
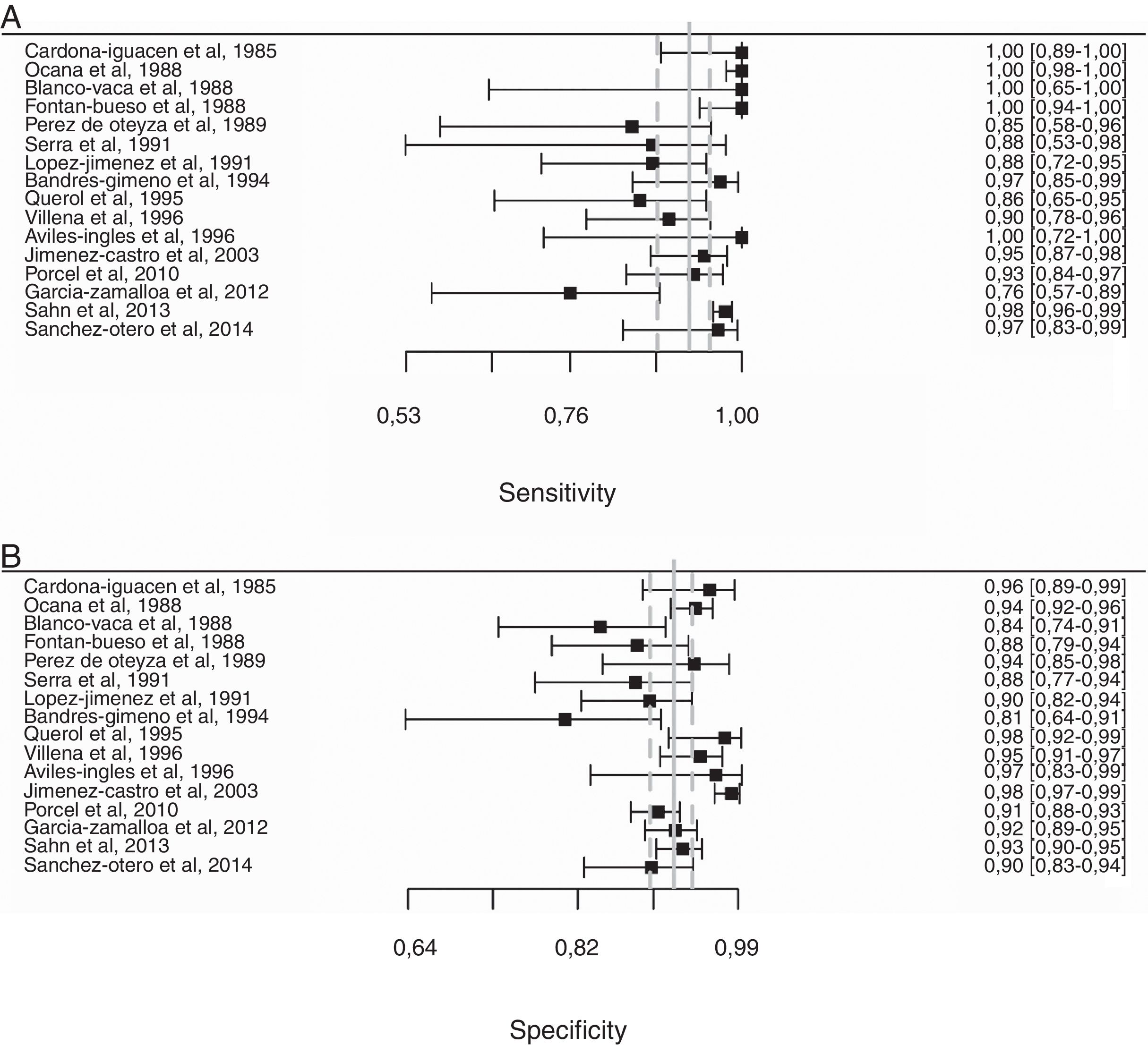
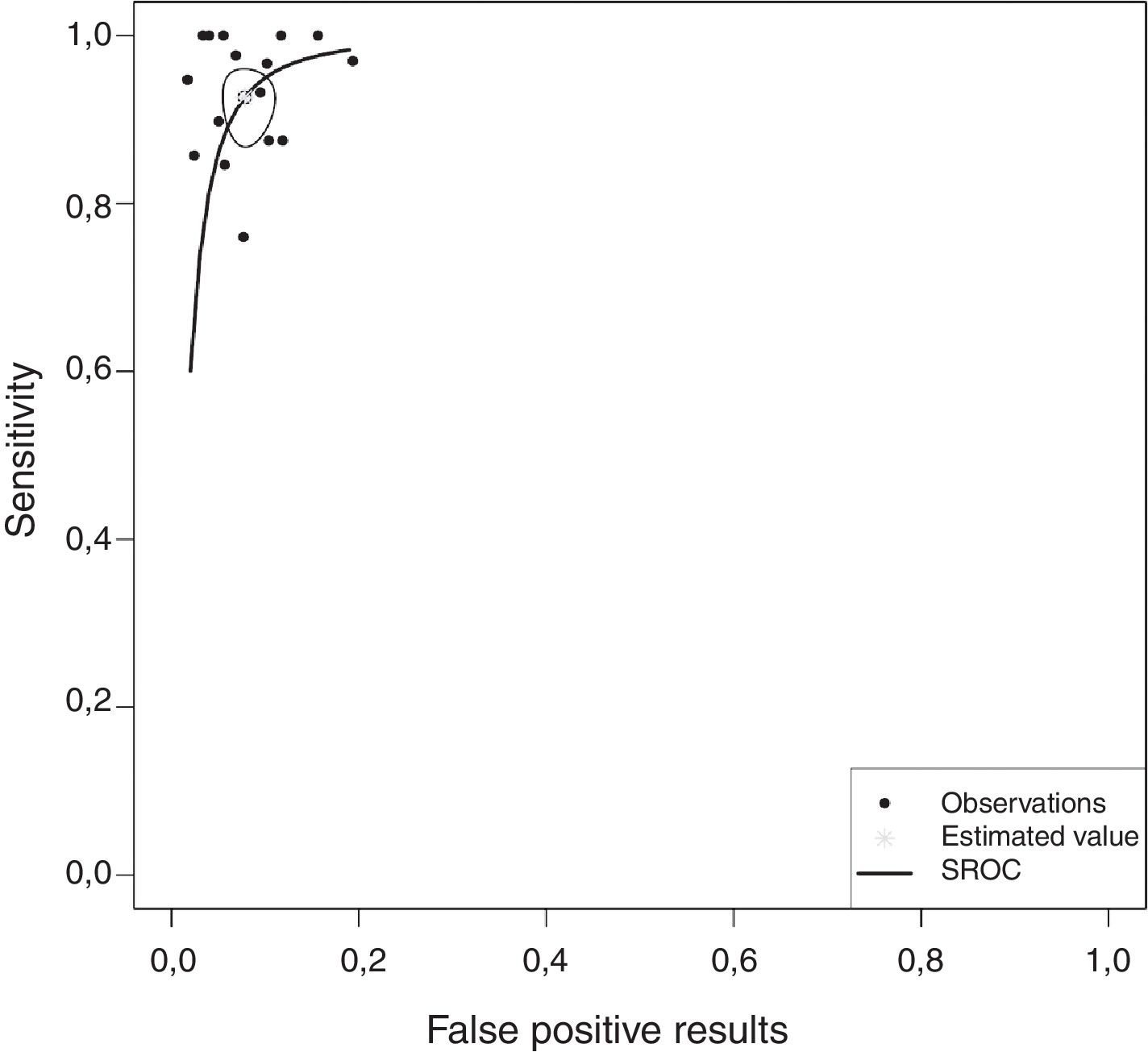
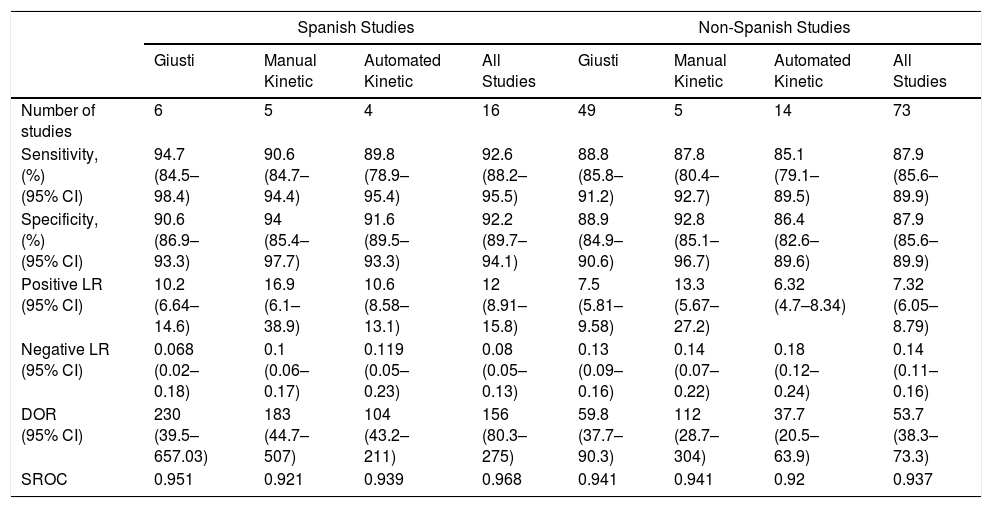
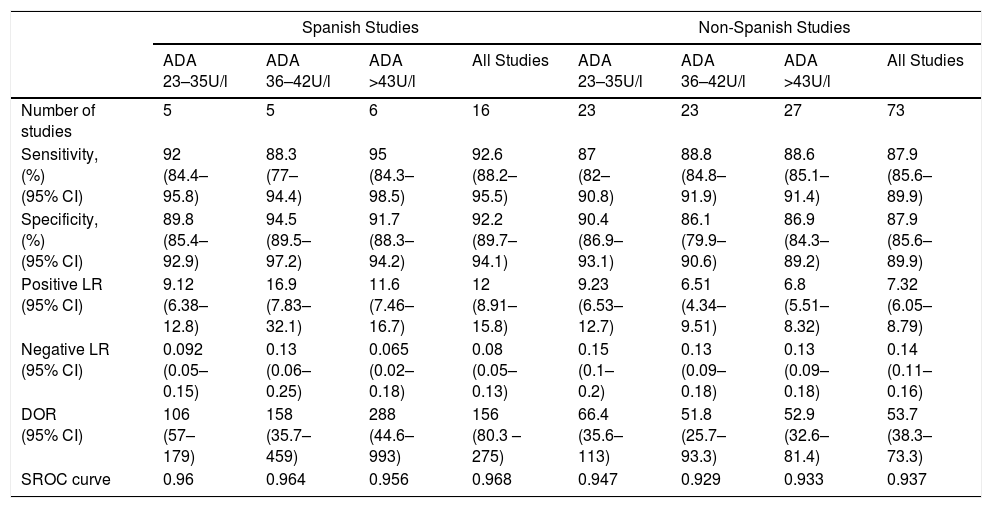
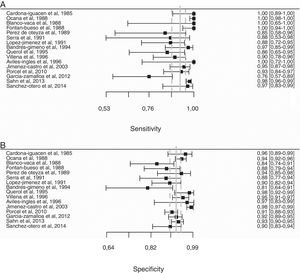
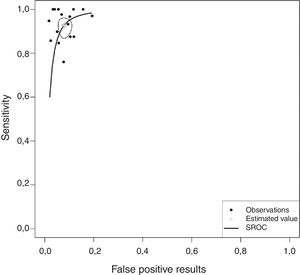
Overall, pleural ADA had a sensitivity of 93% (95% CI: 88%–96%), specificity 92% (95% CI: 90%–94%) (Fig. 2), positive LR of 12 (95% CI: 9–16, negative LR of 0.08 (95% CI: 0.05–0.13), DOR of 156 (95% CI: 80–275) and area under the summary ROC curve of 0.968 (Fig. 3) for identifying TPE. There were no significant differences in sensitivity and/or specificity between different techniques for measuring ADA. Thus, the sensitivity for the Giusti and manual and automated kinetic methods was 95%, 91%, and 90% (P=0.25); and specificity was 91%, 94%, and 92%, respectively (P=0.31) (Table 2). Nor were differences detected in sensitivity or specificity between the different ADA cut-off points, when these were grouped in 3 ranges: (1) ADA 23–35U/l19,22,23,25,28 (sensitivity 92%, specificity 90%); (2) ADA 36–42U/l20,26,27,29,30 (sensitivity 88%, specificity 94%); and (3) ADA 43–45U/l5,16–18,21,24 (sensitivity 95%, specificity 92%; P=0.54 for sensitivity and P=0.1 for specificity).
Forest plot of adenosine deaminase sensitivity (A) and specificity (B) for the diagnosis of tuberculous pleural effusion. The point estimate of sensitivity and specificity of each study is shown as a solid square on a line that represents the confidence interval. The continuous vertical line represents the weighted average sensitivity (A) and specificity (B) and dotted vertical lines represent the confidence interval.
Diagnostic Accuracy of Adenosine Deaminase in Tuberculous Pleural Effusion, According to the Measurement Method Used.
| Spanish Studies | Non-Spanish Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| Giusti | Manual Kinetic | Automated Kinetic | All Studies | Giusti | Manual Kinetic | Automated Kinetic | All Studies | |
| Number of studies | 6 | 5 | 4 | 16 | 49 | 5 | 14 | 73 |
| Sensitivity, (%) (95% CI) | 94.7 (84.5–98.4) | 90.6 (84.7–94.4) | 89.8 (78.9–95.4) | 92.6 (88.2–95.5) | 88.8 (85.8–91.2) | 87.8 (80.4–92.7) | 85.1 (79.1–89.5) | 87.9 (85.6–89.9) |
| Specificity, (%) (95% CI) | 90.6 (86.9–93.3) | 94 (85.4–97.7) | 91.6 (89.5–93.3) | 92.2 (89.7–94.1) | 88.9 (84.9–90.6) | 92.8 (85.1–96.7) | 86.4 (82.6–89.6) | 87.9 (85.6–89.9) |
| Positive LR (95% CI) | 10.2 (6.64–14.6) | 16.9 (6.1–38.9) | 10.6 (8.58–13.1) | 12 (8.91–15.8) | 7.5 (5.81–9.58) | 13.3 (5.67–27.2) | 6.32 (4.7–8.34) | 7.32 (6.05–8.79) |
| Negative LR (95% CI) | 0.068 (0.02–0.18) | 0.1 (0.06–0.17) | 0.119 (0.05–0.23) | 0.08 (0.05–0.13) | 0.13 (0.09–0.16) | 0.14 (0.07–0.22) | 0.18 (0.12–0.24) | 0.14 (0.11–0.16) |
| DOR (95% CI) | 230 (39.5–657.03) | 183 (44.7–507) | 104 (43.2–211) | 156 (80.3–275) | 59.8 (37.7–90.3) | 112 (28.7–304) | 37.7 (20.5–63.9) | 53.7 (38.3–73.3) |
| SROC | 0.951 | 0.921 | 0.939 | 0.968 | 0.941 | 0.941 | 0.92 | 0.937 |
CI, confidence interval; DOR, diagnostic odds ratio; LR, likelihood ratio; SROC, area under the summary ROC curve.
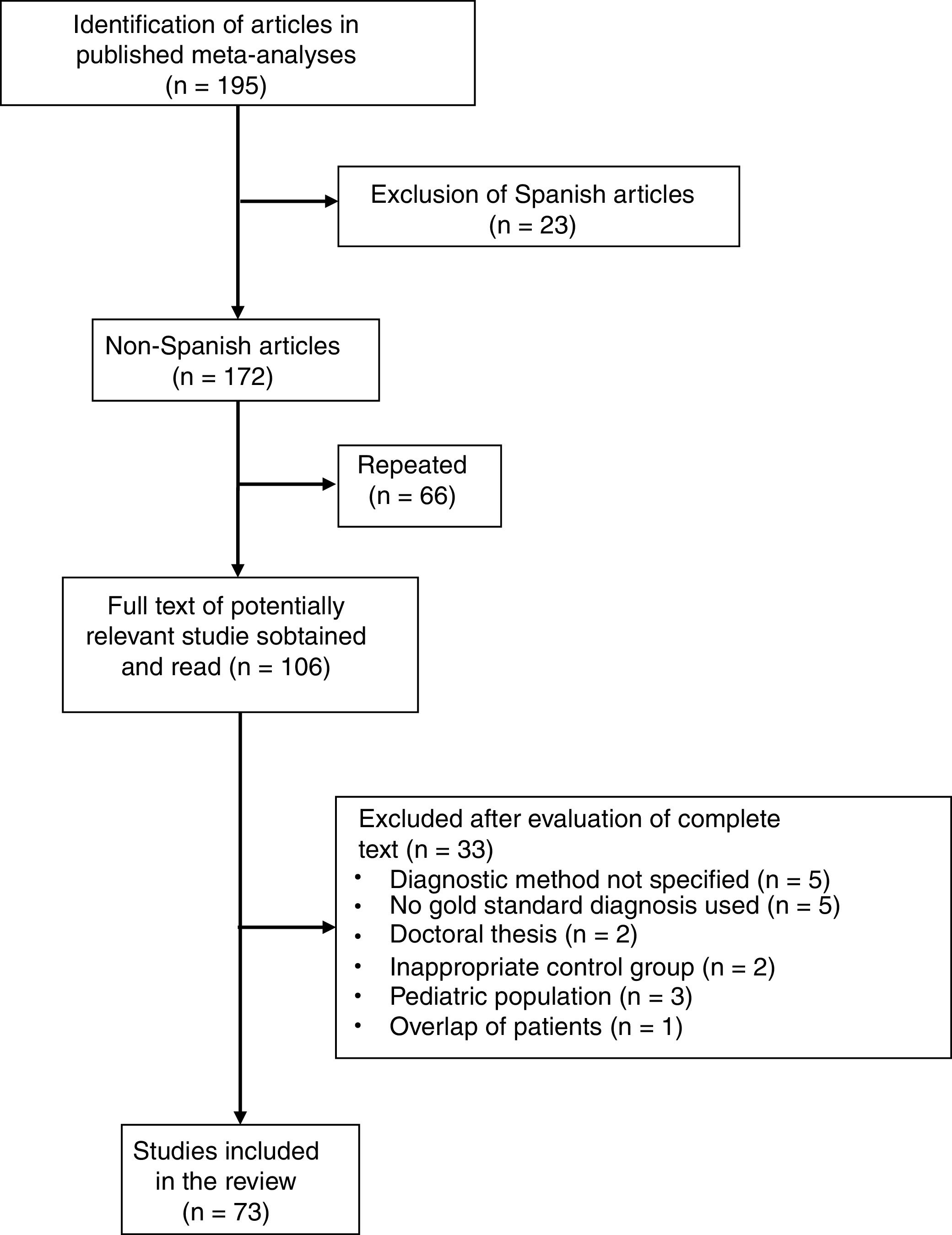
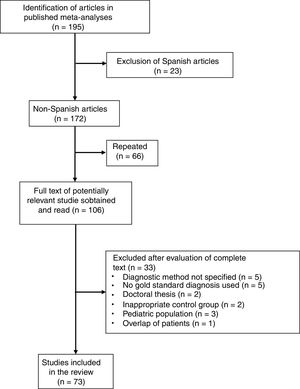
Six meta-analyses were identified,6–11 comprising a total of 195 studies, of which 122 were excluded for the following reasons: 23 because they were performed in a Spanish population (in our meta-analysis, 5 studies were excluded because of duplication of patients and 2 because the results of PF and ascites were combined); 66 due to overlap between the various meta-analyses; 20 due to lack of histological or microbiological confirmation of TPE; 5 because the diagnostic method of the TPE was not specified; 3 that were conducted in the pediatric population; 2 because they were doctoral theses with inaccessible data; 2 because they included an inappropriate comparative group (probable TB); and 1 due to duplication or overlap of patients (Fig. 4 and Table A.1). In this way, 73 articles were selected (Table A.3), including a total of 2789 patients with TPE and 3756 with pleural effusions due to other causes. Among the latter were 63 (1.6%) lymphomas and 614 (16%) parapneumonic effusions, of which 206 were empyemas. The Giusti method was used to quantify ADA in 49 studies, manual kinetic methods in 5, and automated kinetic methods in 14. The technique was not specified in another 5 studies.
Overall, in the non-Spanish studies, pleural ADA showed a sensitivity of 88% (95% CI: 86%–90%), specificity of 88% (95% CI: 86%–90%), positive LR of 7 (95% CI: 6–9), negative LR of 0.14 (95% CI: 0.11–0.16), DOR of 54 (95% CI: 38–73), and area under the summary ROC curve of 0.937 for the diagnosis of pleural TB.
Comparison Between Spanish and Non-Spanish StudiesThere was a non-significant trend toward greater sensitivity (93% vs 88%, P=0.06) and specificity (92% vs 88%, P=0.08) for pleural ADA in the Spanish studies compared to the non-Spanish studies. Sensitivity of the Giusti method (95% vs 89%, P=0.05) and specificity of the automated kinetic techniques (92% vs 86%, P<0.01) were also higher in Spanish studies than in non-Spanish studies. No significant differences were observed for the other techniques.
With regard to the aforementioned ranges of ADA cut-off points, the only factor distinguishing the Spanish and non-Spanish studies was the greater sensitivity of the Spanish studies for the ADA range of 43–45U/l (95% vs 87%, P=0.04), and the greater specificity for ADA values ≥36U/l (ADA 36–42U/l, 95% vs 86%, P=0.04; ADA 43–45U/l, 92% vs 87%; P=0.03, respectively) (Table 3).
Diagnostic Accuracy of Adenosine Deaminase in Tuberculous Pleural Effusion, According to the Cut-Off Point Used.
| Spanish Studies | Non-Spanish Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| ADA 23–35U/l | ADA 36–42U/l | ADA >43U/l | All Studies | ADA 23–35U/l | ADA 36–42U/l | ADA >43U/l | All Studies | |
| Number of studies | 5 | 5 | 6 | 16 | 23 | 23 | 27 | 73 |
| Sensitivity, (%) (95% CI) | 92 (84.4–95.8) | 88.3 (77–94.4) | 95 (84.3–98.5) | 92.6 (88.2–95.5) | 87 (82–90.8) | 88.8 (84.8–91.9) | 88.6 (85.1–91.4) | 87.9 (85.6–89.9) |
| Specificity, (%) (95% CI) | 89.8 (85.4–92.9) | 94.5 (89.5–97.2) | 91.7 (88.3–94.2) | 92.2 (89.7–94.1) | 90.4 (86.9–93.1) | 86.1 (79.9–90.6) | 86.9 (84.3–89.2) | 87.9 (85.6–89.9) |
| Positive LR (95% CI) | 9.12 (6.38–12.8) | 16.9 (7.83–32.1) | 11.6 (7.46–16.7) | 12 (8.91–15.8) | 9.23 (6.53–12.7) | 6.51 (4.34–9.51) | 6.8 (5.51–8.32) | 7.32 (6.05–8.79) |
| Negative LR (95% CI) | 0.092 (0.05–0.15) | 0.13 (0.06–0.25) | 0.065 (0.02–0.18) | 0.08 (0.05–0.13) | 0.15 (0.1–0.2) | 0.13 (0.09–0.18) | 0.13 (0.09–0.18) | 0.14 (0.11–0.16) |
| DOR (95% CI) | 106 (57–179) | 158 (35.7–459) | 288 (44.6–993) | 156 (80.3 – 275) | 66.4 (35.6–113) | 51.8 (25.7–93.3) | 52.9 (32.6–81.4) | 53.7 (38.3–73.3) |
| SROC curve | 0.96 | 0.964 | 0.956 | 0.968 | 0.947 | 0.929 | 0.933 | 0.937 |
ADA, adenosine deaminase; CI, confidence interval; DOR, diagnostic odds ratio; LR, likelihood ratio; SROC, area under the summary ROC curve.
With regard to the etiology of non-tuberculous effusions, more lymphomas were found in the Spanish studies (78/2975, 2.7% vs 63/3756, 1.7%; P=0.01), and more empyemas in the non-Spanish studies (206/3756, 5.5% vs 75/2975, 2.5%; P<0.01). The percentage of lymphomas and empyemas taken together was higher in the non-Spanish studies (7.2% vs 5.2%, P<0.01).
Risk of Bias and HeterogeneityThe asymmetric funnel test for LR showed no significant publication bias in the Spanish studies (P=0.46 for positive LR; P=0.62 for negative LR), or when the ADA measurement techniques or their different cut-off points were considered individually (all P>0.1). There was no significant heterogeneity in the set of Spanish studies (I2=20% for positive LR; I2<13% for negative LR and DOR), or for those conducted using the Giusti (I2=0%), manual kinetic (I2=0%) or automated kinetic methods (I2=0.2%), or when ADA cutoff points of <36U/l (I2<17.1%), between 36 and 42U/l (I2=0%) or > 42U/l (I2<17.3%) were used.
DiscussionThis meta-analysis demonstrates the high yield of pleural ADA in the diagnosis of TB in the Spanish population. High concentrations (generally ≥35–40U/l) significantly increase the likelihood of TPE (positive LR=12), while low values reduce it (negative LR=0.08). Neither the various ADA measurement techniques (Giusti and manual or automated kinetic methods) nor the various diagnostic cut-off points for TB described in the literature influenced the diagnostic efficacy of this enzyme. In studies in non-Spanish populations, pleural ADA showed lower sensitivity (88% vs 93%) and specificity (88% vs 92%) than in the Spanish population, although the differences did not reach statistical significance.
Diseases other than TB can be accompanied with elevated pleural ADA. A series of 2100 patients with pleural effusion reported that up to 70% of empyemas and about half of lymphomas had ADA concentrations in PF≥35U/l.28 In this meta-analysis, we found that the percentage of combined empyemas and lymphomas was higher in non-Spanish studies (7.2% vs 5.2%). This finding may be explained by the trend toward greater ADA specificity in the non-Spanish population.
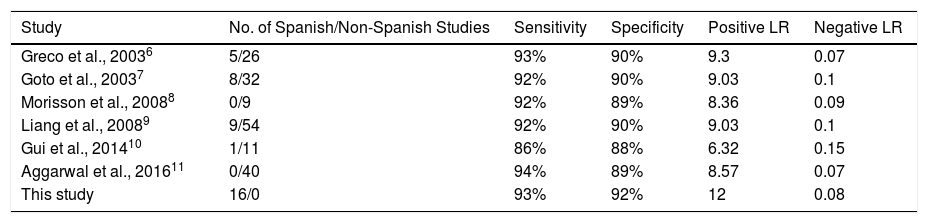
Sensitivity and specificity figures for pleural ADA in the Spanish studies were comparable to those reported in other meta-analyses (Table 4). However, some of these show deficiencies that we have attempted to resolve in this study. For example, 3 did not evaluate positive LR, negative LR or DOR.6–8 Of 172 non-Spanish studies extracted from the 6 published meta-analyses, only 73 met our inclusion criteria (42%) (Table 4 and Table A.2). Some of the reasons for exclusion (e.g., no gold standard diagnosis of TPE in 20 studies) might raise questions regarding the strength of the results obtained in those meta-analyses.
Diagnostic Accuracy of Adenosine Deaminase in Tuberculous Pleural Effusion, According to Different Published Meta-analyses.
| Study | No. of Spanish/Non-Spanish Studies | Sensitivity | Specificity | Positive LR | Negative LR |
|---|---|---|---|---|---|
| Greco et al., 20036 | 5/26 | 93% | 90% | 9.3 | 0.07 |
| Goto et al., 20037 | 8/32 | 92% | 90% | 9.03 | 0.1 |
| Morisson et al., 20088 | 0/9 | 92% | 89% | 8.36 | 0.09 |
| Liang et al., 20089 | 9/54 | 92% | 90% | 9.03 | 0.1 |
| Gui et al., 201410 | 1/11 | 86% | 88% | 6.32 | 0.15 |
| Aggarwal et al., 201611 | 0/40 | 94% | 89% | 8.57 | 0.07 |
| This study | 16/0 | 93% | 92% | 12 | 0.08 |
LR, likelihood ratio.
This meta-analysis is the first to evaluate whether the different ADA measurement techniques have similar diagnostic efficacy. No differences were observed between these methods in the Spanish population. However, when the Spanish and non-Spanish populations were compared, ADA was more sensitive among the Spanish population when it was analyzed using the Giusti method (95% vs 89%, P=0.05), and more specific when automated kinetic techniques were used (92% vs 86%, P<0.01).
Our study has some limitations. We focused on the Spanish population, in order to reduce the effect that the prevalence of TB among the different populations might have on the diagnostic efficacy of ADA, but prevalence also varies among the different regions of Spain. In fact, most of the studies included in this meta-analysis come from the autonomous communities of Catalonia,16–18,21,28 Galicia,5,19,23,30 and Madrid.20,22,25,27 Moreover, the studies were performed over a long period, during which the prevalence of TB varied.31 Specifically, in the 3 Spanish communities mentioned above, the prevalence of TB was fell from 41, 71, and 30 cases per 100000 inhabitants in 1997.32,33 to 15, 20, and 10 cases per 100000 inhabitants in 2014, respectively.2 Other sources of heterogeneity were also detected, such as the inclusion of patients with non-tuberculous effusions, and effusion due to poorly defined or idiopathic disease. Nevertheless, we were able to use the meta-regression analysis to control for factors such as the different techniques for measuring ADA or the choice of different ADA cut-off points described in the medical literature. Finally, as this was not a meta-analysis of individual data, the effect of factors such as age on the ideal cut-off point for pleural ADA in the diagnosis of TPE could not be evaluated. Some retrospective studies suggest that dichotomous cut-off points should be adopted, with lower values for older patients (>45–55 years).34–36
In conclusion, this study resolves some of the methodological deficiencies of previous meta-analyses, and shows that pleural ADA is a precise method for diagnosing TPE in the Spanish population, irrespective of the measurement technique used for analysis.
Conflict of interestThe authors state that they have no conflict of interest.
Please cite this article as: Palma RM, Bielsa S, Esquerda A, Martínez-Alonso M, Porcel JM. Eficacia diagnóstica de la adenosina desaminasa en líquido pleural para diagnosticar tuberculosis. Metaanálisis de estudios españoles. Arch Bronconeumol. 2019:55:23–30.