Journal Information
Vol. 60. Issue 12.
Pages 733-734 (December 2024)
Share
Download PDF
More article options
Vol. 60. Issue 12.
Pages 733-734 (December 2024)
Editorial
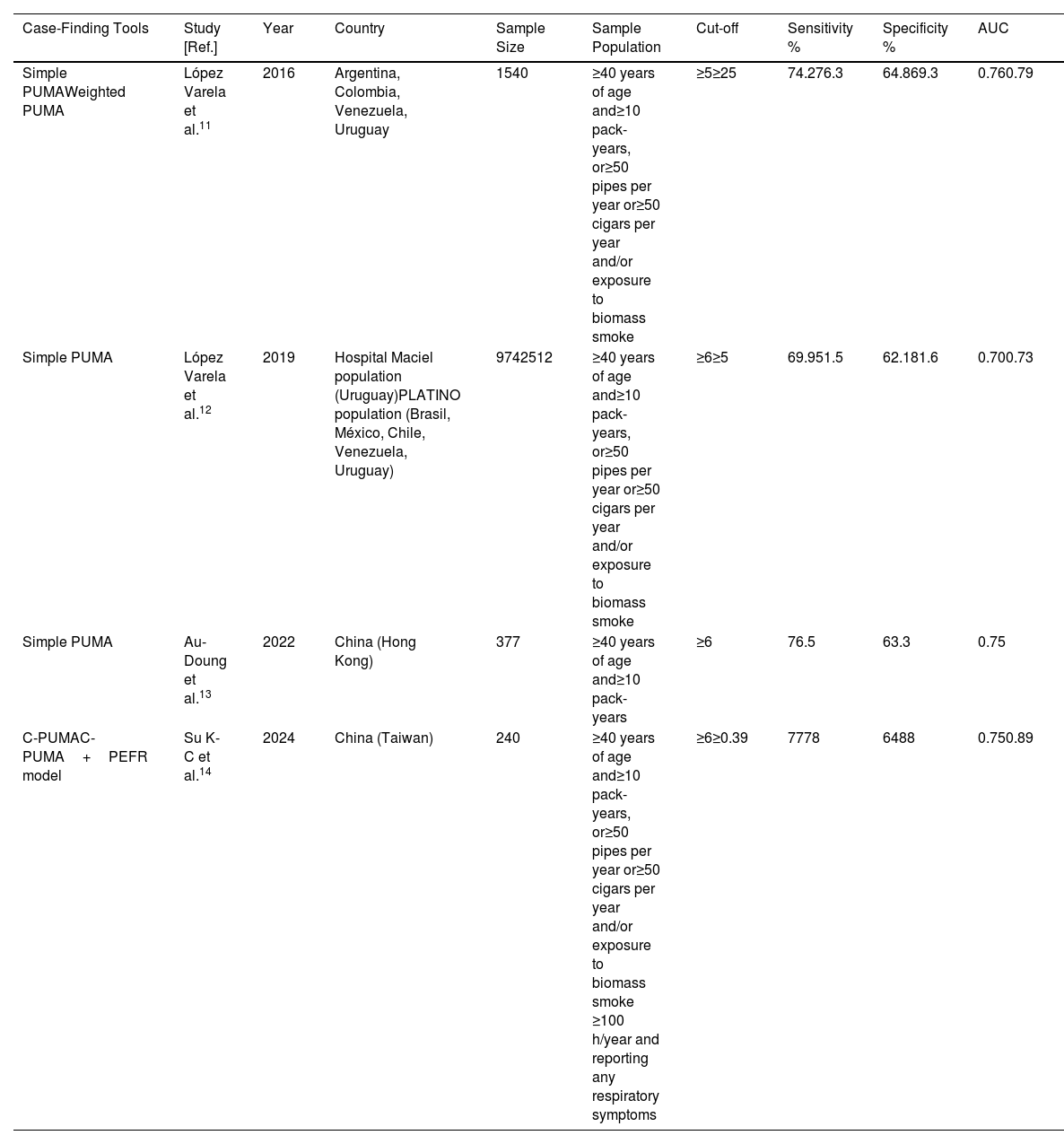
Diagnostic Accuracy of the Combined Modality of a Questionnaire and a Portable Device for COPD Case Finding: Experience With the PUMA Questionnaire
Visits
529
This item has received
Article information
These are the options to access the full texts of the publication Archivos de Bronconeumología