In the last 2 decades, drug-resistant tuberculosis has become a threat and a challenge to worldwide public health. The diagnosis and treatment of these forms of tuberculosis are much more complex and prognosis clearly worsens as the resistance pattern intensifies. Nevertheless, it is important to remember that with the appropriatesystematic clinical management, most of these patients can be cured.
These guidelines itemize the basis for the diagnosis and treatment of all tuberculosis patients, from those infected by strains that are sensitive to all drugs, to those who are extensively drug-resistant. Specific recommendations are given forall cases. The current and future role of new molecular methods for detecting resistance, shorter multi-drug-resistant tuberculosis regimens, and new drugs with activity against Mycobacterium tuberculosis are also addressed.
En las últimas 2 décadas la tuberculosis con resistencia a fármacos se ha convertido en una amenaza y un reto para la salud pública mundial. El diagnóstico y el tratamiento de estas formas de tuberculosis es mucho más complejo, y el pronóstico empeora claramente a medida que se incrementa el patrón de las resistencias. Sin embargo, es necesario destacar cómo con el manejo clínico y programático adecuado de estos enfermos se puede conseguir la curación de una mayoría de ellos.
En esta normativa se razonan las bases del diagnóstico y tratamiento de todos los pacientes afectos de tuberculosis, desde aquellos que tienen formas de la enfermedad con sensibilidad a todos los fármacos hasta aquellos que son portadores de los patrones más extensos de resistencia. Asimismo, se dan recomendaciones específicas para cada uno de estos supuestos. También se aborda el papel que ya están teniendo y pueden tener en un futuro inmediato los nuevos métodos moleculares de detección de resistencias, los esquemas acortados de tratamiento de la tuberculosis multi-farmacorresistente (TB-MDR) y los nuevos fármacos con actividad frente a Mycobacterium tuberculosis.
After decades during which tuberculosis (TB) became an almost universally curable disease, the emergence of strains of Mycobacterium tuberculosis (M. tuberculosis) resistant to the most active drugs available has once again made TB a major threat and a challenge to global public health.1 It is, however, important to remember that if access to appropriate diagnosis and treatment are guaranteed, the chances of curing all patients with TB are very high, even among carriers of highly resistant strains. Protocolized clinical management is essential if the treatment of these patients is to be successful.2–9
The current global status of multidrug-resistant TB (MDR-TB) – TB resistant to at least isoniazid (H) and rifampicin (R) – is worrying, and the overall response to this situation has been unsatisfactory.1,10 An estimated 3.9% of new TB cases worldwide are thought to be MDR-TB, and this rate rises to 21% in previously treated patients.1 Of the estimated 10.6 million cases of TB occurring in 2015 (1.8 million deaths), about 580000 cases may have been rifampicin-resistant (RR-TB) or MDR-TB, leading to around 250000 deaths.1 Worldwide, however, less than 150000 cases (26% of the estimated total) were reported, with a cure rate of 52%.1 In other words, only about 10% of the estimated MDR-TB patients worldwide were cured, a rate that is totally ineffective for controlling the epidemic.
Although the prevalence of MDR-TB in Spain remains difficult to pinpoint due to underreporting of TB and failure to perform systematic resistance testing, the situation seems more favorable: 0.1% cases of TB in Spanish natives and 2.2% in immigrants were primary MDR-TB, of which 3.4% and 10.2%, respectively, showed primary resistance to H.11,12
Diagnosis of Drug-Resistant TuberculosisTB diagnosis is still based on clinical suspicion, radiology, and microbiological testing.13 The clinical and radiological features of drug-resistant tuberculosis (DR-TB) are indistinguishable from those of drug-susceptible TB, so diagnosis of DR-TB must always be based on microbiological and/or molecular evidence.6
Drug Susceptibility StudiesSpanish guidelines currently recommend cultures and susceptibility studies, including at least isoniazid (H) and rifampicin (R), in all patients with TB, irrespective of whether the patient is treatment-naïve or presents a risk factor for DR-TB (Table 1)3,6,16,17 (strong recommendation, high quality of evidence [⊕⊕⊕⊕]).
Risk Factors for Drug-Resistant Tuberculosis.3,6,16
| High risk factors |
| Patients previously treated for TB, particularly those who failed previous treatment regimens, but also relapsers and drop-outs resuming treatment |
| Cohabitant or close contact of an MDR-TB patient |
| Moderate risk factors |
| Patients with positive sputum smear at the end of the second month of initial treatment (HRZE) and in whom initial drug susceptibility is unknown |
| Patients from countries with high rates of initial MDR-TB |
| Patients who live in closed institutions, such as prisons or hostels where there have been cases of MDR-TB |
| Healthcare personnel, particularly those who treat MDR-TB cases |
| Patients with comorbidities that may lead to situations of malabsorption |
| HIV infection |
If resistance to R is shown, the susceptibility study should be extended to include the fluoroquinolone (FQ) and the second-line injectable drug (SLID) that will be used in the rescue treatment offered to the patient6 (levofloxacin [Lfx]/moxifloxacin [Mfx] and amikacin [Am]/capreomycin [Cm] in Spain) (conditional recommendation, moderate quality of evidence [⊕⊕
]).The results of conventional susceptibility testing to all these drugs are very reliable, so this information can be used to guide the recommended treatment regimen. In contrast, the clinical credibility of susceptibility testing to other drugs such as ethambutol (E), pyrazinamide (Z), ethionamide/prothionamide (Eth/Pth), cycloserine (Cs), para-aminosalicylic acid (PAS), or clofazimine (Cfz) is significantly lower, and these results can often confound rather than assist decisions regarding the possible treatment regimen needed by the patient. For this reason, routine testing is not recommended, and if these tests are performed, the results must be viewed with caution5,6,18 (conditional recommendation, low [⊕
] to very low [] quality of evidence).Phenotypic and Genotypic Drug Susceptibility TestingSusceptibility tests can be performed using phenotypic or genotypic methods. Phenotypic testing must be performed on mycobacteria in active growth phase in the culture media, so results will be unavailable for at least 2–3 weeks if liquid media are used, and up to 4–8 weeks in the case of solid media.6 This delay may be excessive if a decision is to be made regarding the ideal treatment of the patient.
In contrast, molecular tests, which use genetic amplification techniques to detect mutations in genes coding for resistance to anti-TB drugs, provide results within 24–48h. For this reason, such tests, when available, should be performed in all patients diagnosed with TB (strong recommendation, high quality of evidence [⊕⊕⊕⊕]).
Specific Molecular Techniques: Xpert MTB/RIF (Cepheid) and GenotypeMDRplus (Hain)One of the most important of these molecular techniques is the Xpert MTB/RIF (Cepheid) that can detect resistance to R within 2h with a sensitivity of 95% and a specificity of 98%.14,17 This technique is much more sensitive than sputum smears (positive in up to 70%–90% of cases with negative smear test and positive culture), and represents an important advance in early detection. The GenotypeMDRplus (Hain) or line probe assay can also simultaneously detect mutations in genes that encode resistance to isoniazid (katG and inhA) and rifampicin (rpoB) within a period of 6–24h.19,20
Both molecular techniques can be carried out in direct samples, without the need to wait for the isolate to grow in culture. The clinical significance of the inh A gene mutation and/or the katG gene and the recommendation to use H despite proven resistance are described in detail in the online version of this guideline.21–28
Version 2 of the GenotypeMDRsl (Hain) line probe assay can be used to detect resistance to FQ (gyrA and gyrB mutations) and to SLID (genetic mutations in rrs and the eis promoter region). The specificity of this method for both FQs and SLID is higher than 98%,29–31 so this information should be taken into consideration in the design of the treatment schedule32,33 (conditional recommendation, moderate quality of evidence [⊕⊕
]).Clinical History of Previously Administered DrugsIt is of utmost importance to consult the clinical records of patients previously treated for TB to determine their drug history.6 When the wrong drug has been administered for more than 1 month, the possibility of resistance and reduced efficacy must be suspected, even if the susceptibility testing suggests the opposite.6,34 There are simple drug history templates that can help with this task, as shown in Fig. 1.6 It is also necessary to determine if the patient's index case had DR-TB.
Template for collecting patient anti-TB drug history. Adapted from Caminero et al.6
This guideline recommends that: (1) all patients with diagnosis of TB undergo H and R susceptibility testing, using a rapid test molecular if available; (2) if resistance to R and/or H is demonstrated, susceptibility tests to the FQ and SLID proposed for the subsequent treatment schedule should be performed, using GenotypeMDRsl (Hain) v2 if possible; (3) standard phenotypic tests must also be performed; while these take longer and support the treatment decision to a lesser degree, they can resolve any discrepancies between the methods; and (4) a clinical drug history is essential for designing future treatment regimens.
Principles for the Treatment of Both Drug-Susceptible and Drug-Resistant Forms of TuberculosisAll TB treatment must meet 2 basic bacteriological requirements: drugs must be combined to prevent the selection of resistance and the treatment must be administered for long enough to ensure cure and avoid relapses.6,35,36
To ensure cure of TB without relapse as far as possible, it is recommended that all treatment regimens involve a combination of at least 4 previously unused drugs or drugs to which the M. tuberculosis has shown susceptibility.6,15,36 Of these, 2 must be core drugs, capable of eliminating the majority of the bacilli and curing the patient. At least 1 of these core drugs must have good bactericidal activity (ability to eliminate rapidly multiplying bacilli located in cavitated lesions that cause symptoms and transmission), and at least 1 must have good sterilizing activity (ability to eliminate those bacilli in semilatent phases that are responsible for relapses). The other 2 drugs are what we call companion compounds, and their mission is to protect the core drugs from selection of resistance6,36 (conditional recommendation, moderate quality of evidence [⊕⊕
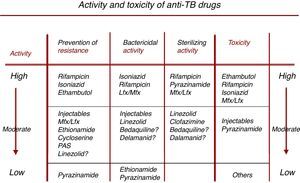
]).To assist in the selection of the drugs needed to make up a TB regimen, Fig. 2 shows the bactericidal and sterilizing capacity of the different drugs, their ability to prevent the selection of resistance, and their toxicity profile.36
Characteristics of drugs with activity against M. tuberculosis. Adapted from Caminero et al.36
Prevention of resistance, bactericidal activity and sterilizing activity are listed in descending order (high, moderate, and low activity), while toxicity (right-hand arrow) is listed in ascending order (low, moderate, high), so that the best available drugs combining all these features appear in the top row.
Tables 2a and 2b list in detail the doses of the different drugs with activity against M. tuberculosis, the route of administration, and the most common adverse effects.
Drugs With Anti-M. tuberculosis Activity. Recommended Doses and Most Common Adverse Effects (a, Recommended Doses for Each of the Drugs Used Individually).
| Drug | Route | Dose | Most Common Adverse Effects |
|---|---|---|---|
| Rifampicin | Oral, IV | 10mg/kg. Max. 600mg | Hepatitis, hypersensitivity reactions |
| Isoniazid | Oral, IV, IM | 5mg/kg at normal doses (max. 300mg) 15mg/kg at high doses | Hepatitis. Peripheral neuritis |
| Pyrazinamide | Oral | 25–30mg/kg | Hepatitis. Hyperuricemia |
| Ethambutol | Oral | 25mg/kg 15mg/kg in continuation phase | Optic neuritis |
| Streptomycin | IM, IV | 15mg/kg. Max. 1g | Nephrotoxicity, 8th cranial nerve involvement |
| Ethionamide/prothionamide | Oral | 750–1000mg | Gastroenteritis/hepatitis |
| Cycloserine | Oral | 750–1000mg | Personality changes/depression |
| Capreomycin | IM, IV | 15mg/kg Max. 0.75–1g/day or/48h | Ototoxicity/nephrotoxicity |
| Kanamycin | IM, IV | 15mg/kg Max. 0.75–1g/24h or/48h | Ototoxicity/nephrotoxicity |
| Amikacin | IM, IV | 15mg/kg Max. 0.75–1g/24h or 48h | Ototoxicity/nephrotoxicity |
| Levofloxacin | Oral, IV | 15mg/kg→750mg−1g | Tenosynovitis |
| Moxifloxacin | Oral | 400–800mg | Tenosynovitis |
| PAS | Oral | 10–15g | Gastroenteritis/hepatitis |
| Clofazimine | Oral | 100–200mg | Pigmentation/eosinophilic enteritis |
| Linezolid | Oral, IV | 600mg | Pancytopenia/gastrointestinal disorders/polyneuritis |
| Meropenem | IV | 1g/8h or 12h | Hematological disorders |
| Bedaquiline | Oral | 400mg/day for 15 days and then 200mg/3 times a week for a maximum of 6 months | Gastric intolerance, pancreatitis, hepatitis, altered QTc on ECG |
| Delamanid | Oral | 100mg/12h for a maximum of 6 months | Anemia, nausea, altered QTc on ECG |
Drugs With Anti-M. tuberculosis Activity. Recommended Doses and Most Common Adverse Effects (b, Recommended Doses for Combinations of the Most Common Drugs Available in Spain).
| Combination isoniazid+rifampicin (H+R) | |
| Rifinah 300®(H 150mg, R 300mg) | |
| Patients weighing 30–50kg | 1tab./day |
| Patients 50kg or more | 2tab./day |
| Combination isoniazid+rifampicin+pyrazinamide (H+R+Z) | |
| Rifater®(H 50mg, R 120mg, Z 300mg) | |
| In children under the age of 10 years, adjust according to dose | |
| Patients weighing 20–30kg | 2tab./day |
| Patients weighing 30–40kg | 3tab./day |
| Patients weighing 40–49kg | 4tab./day |
| Patients weighing 50–64kg | 5tab./day |
| Patients 65kg or more | 6tab./day |
| Combination isoniazid+rifampicin+pyrazinamide+ethambutol (H+R+Z+E) | |
| Rimstar®(H 75mg, R 150mg, Z 400mg, E 275mg) | |
| Patients weighing 30–39kg | 2tab./day |
| Patients weighing 40–54kg | 3tab./day |
| Patients weighing 55–70kg | 4tab./day |
| Patients 70kg or more | 5tab./day |
To assist in the selection of the 4 drugs that must be included in any treatment of initial phase TB, these compounds should be classified into 5 different groups (Table 3), starting with Group 1, the group with the greatest activity, and continuing with the others in decreasing order of effectiveness and tolerance.3,8,37,38 Prescribing physicians should work their way down through these groups until at least 4 new drugs or drugs very likely to be susceptible have been combined, ensuring the inclusion of at least 1 bactericidal drug and 1 sterilizing drug. Guidelines for starting each of the drugs included in these groups are shown in Table 3 (conditional recommendation, moderate quality of evidence [⊕⊕
]).Rational Classification and Sequential Use of Anti-TB Drugs When Designing a Treatment Regimen for Drug-Susceptible or Drug Resistant TB.
| First-line drugs for oral administrationa |
| Core drugs: isoniazid, rifampicin, pyrazinamide |
| Companion drug: ethambutol |
| Fluoroquinolonesb |
| High-dose levofloxacin or moxifloxacin, all are core |
| Second-line injectable drugsb |
| Streptomycinc, kanamycin, amikacin, capreomycin, all are core |
| Mixed group of core drugs with little evidence and less effective companion drugsd |
| core drugs: linezolid, bedaquiline, delamanid |
| companion drugs: clofazimine, prothionamide/ethionamide, cycloserine/terizidone |
| Other drugs with less clinical experience, or less effective and more toxice |
| Carbapenems (meropenem/imipenem)+amoxicillin/clavulanic acid, PAS, thioacetazone |
Sources: World Health Organization4; Caminero et al.6; World Health Organization8; Caminero et al.37; Caminero et al.38
Use only 1, because the genetic target is the same. Consider it as active drug in MDR-TB cases. In XDR-TB cases, add a fluoroquinolone and/or an injectable if in vitro susceptibility to any of these drugs is maintained, and always try to ensure it is different from the previously used compound. In XDR-TB, these products should not be included among the 4 active agents of the regimen.
Avoid streptomycin due to its high rate of resistance associated with isoniazid, but it may be considered if it is seen to be susceptible on resistance testing, and if it has not been previously used in the patient.
Group 1 consists of “first-line oral drugs”, so called because these are used in the first instance in practically all patients with drug-susceptible TB. This group comprises the most effective, the best tolerated, and the least expensive products.37,38 Drugs classified as core because of their bactericidal (H and R) or sterilizing (R and Z) activity must be distinguished from companion drugs (E).6,36
Group 2 consists of the FQs (high-dose Lfx or Mfx), which are also core drugs with bactericidal and sterilizing activity and low toxicity.6,37,38 Moreover, if these products can be used, they clearly affect prognosis in the treatment of MDR-TB.6,39,40 Although no specific studies have been performed, evidence suggests the presence of considerable cross-resistance between these 2 FQs.
Group 3 consists of second-line injectable drugs. These are also core drugs, due to their bactericidal activity, but with little or no sterilizing ability and they are far more toxic than the FQs.6,37,38 This cumulative toxicity and the need for administration by injection greatly limit their use.
Group 4 is composed of 6 different drugs that might be administered in combination because they attack M. tuberculosis via different targets.3,4,6,37,38 This is a mixed group consisting of drugs that could be considered core due to their activity, although the accumulated evidence is still scant (linezolid, bedaquiline and delamanid), and others that have stronger supporting evidence but moderate or low activity (ethionamide/prothionamide and cycloserine/terizidone) and would behave as companion drugs, and a third, clofazimine, which seems to have good sterilizing activity. The selection of one or the other will depend on their availability and possible adverse effects.
Linezolid can be considered a core drug, with bactericidal and sterilizing activity.38 Several publications38,41–43 have confirmed its role in the treatment of MDR-TB and extensively drug resistant TB (XDR-TB). Linezolid has 2 drawbacks, namely its cost and its toxicity profile when administered for more than 6–8 weeks, with frequent hematological alterations and polyneuropathies. Toxicity problems are highly dose-dependent38 and relatively easy to manage, given the low dose recommended for MDR-TB,38,41 while the price has fallen since the generic molecule became available in 2016.
Bedaquiline can also be considered core due to its bactericidal and sterilizing activity.38,44 Two clinical trials have demonstrated its efficacy in the treatment of MDR-TB,45,46 particularly in XDR-TB (MDR-TB resistant to at least 1 FQ and 1 SLID [kanamycin, amikacin, capreomycin]), and it is used in many countries.
Finally, delamanid and pretomanid are metronidazole derivatives with bactericidal and sterilizing activity.38,47–51 Two randomized clinical trials have demonstrated the usefulness of delamanid in the treatment of MDR-TB,48,49 particularly in XDR-TB.
Linezolid, bedaquiline, and delamanid are all likely to play a significant role in the treatment of MDR-TB in the very near future,38 and may also be of use in the treatment of susceptible TB.
Aside from the foregoing drugs, the most effective compounds are the thioamides (ethionamide/prothionamide). These may be mildly bactericidal,6 but show potential cross-resistance with H (inhA gene mutation, detectable by GenotypeMDRplus) and a poor gastric tolerance profile.6 The next most active is clofazimine, an important component of short treatments of MDR-TB due to its possible sterilizing activity.8,26,52 The last is cycloserine (similar to terizidone in terms of action), which has good oral tolerance, but limited activity and potentially serious psychiatric adverse effects.6,53
A hypothetical Group 5 would include the carbapenems (imipenem or meropenem) combined with clavulanic acid (as this does not exist separately, it must be administered with amoxicillin), which are probably quite active despite the scant supporting evidence6,54; PAS, which is very ineffective, with poor gastric tolerance6,37; and thioacetazone, which is very weak, potentially toxic in patients with human immunodeficiency virus (HIV), and very difficult to obtain.37
Treatment of Tuberculosis According to Resistance PatternsTB, according to possible resistance patterns, difficulty of treatment and the different prognoses involved, can be classified into 5 large groups in ascending order of complexity55 (Table 4).
Recommended Basic Regimens for Patients With Susceptible TB and Single or Multiple Resistances.
| Initial TB cases susceptible to all drugsa,b,c |
| 2HRZE/4(HR) |
| TB cases resistant to H (single or multiple resistances), but susceptible to Rd,e |
| 9HRZE, or 2FQ-REZ/7FQ-RE or 2RZE/10RE |
| Cases resistant to R (single or multiple resistances) but susceptible to H, or if susceptibility to H is unknown |
| Same treatment as MDR-TB, which is shown in Table 5, adding H to the regimen, but without counting it as one of the 4 new drugs |
There is no consensus with regard to the duration of this treatment in HIV-infected patients, and some groups and scientific societies recommend continuing treatment for 9 months, with the aim of reducing the relapse rate.
Do not switch to the continuation phase (4 HR) until 1 of the following 2 circumstances occurs: sputum smear is negative, or susceptibility to H and R is confirmed.
Prolong treatment beyond 6 months in patients in whom sputum smears and/or cultures take longer than 2 months to become negative.6 As a reference, these patients will continue treatment with H+R for at least 4 months after cultures become negative.
FQ (Lfx/Mfx) should only be included in the regimen if it is administered with the rest of the drugs from the beginning. It should not be added if results of resistance testing to H are received after 3–4 weeks of treatment, due to the possible risk of de facto monotherapy. In this case, use 9 HRZE.
The ideal treatment regimen for new TB cases in which susceptibility to all drugs is assumed would be 2HRZE/4HR, taking into consideration the factors listed in Table 46,13,15,36,56 (strong recommendation, high quality of evidence [⊕⊕⊕⊕]). To reduce the possibility of errors and selection of resistances, these drugs should be administered in fixed dose combinations (Tables 2a and 2b) with directly observed therapy in patients with risk factors for poor treatment compliance.15
Treatment of Tuberculosis Resistant to Isoniazid (Single or Multiple Resistances) but Susceptible to RifampicinThis is a relatively common situation. In these cases, the recommendation is to use 9 HRZE (in this case, high doses of H may be considered),23 and to perform a susceptibility study with the remaining first-line drugs (conditional recommendation, low [⊕
] to very low () quality of evidence). A regimen containing 2FQ-REZ/7CF-RE could also be considered (with an FQ susceptibility study), but FQ (Lfx/Mfx) should only be included if it is administered from the beginning of treatment along with the other drugs. It should not be added if results of resistance testing to H are received after 3–4 weeks of treatment, due to possible risk of de facto monotherapy. See options in Table 4.4,6,15 A third possibility, in line with current SEPAR recommendations is 2RZE/10RE, with has been used successfully in standard treatments when H had to be discontinued due to intolerance.Treatment of Tuberculosis Resistant to Rifampicin (Single or Multiple Resistances), but Susceptible to IsoniazidAs cases of isolated resistance to R are very rare in clinical practice, and since R is the compound that determines prognosis in patients with MDR-TB, these patients should be approached as MDR-TB cases, and treated as such, with the addition of H to the schedule, of course, because if susceptibility is confirmed, this will contribute significantly to the treatment5,6 (conditional recommendation, low [⊕
] to very low [] quality of evidence).Treatment of Multi-Drug Resistant Tuberculosis Susceptible to Fluoroquinolones and Second-Line Injectable DrugsFollowing the rationale of this guideline, the ideal regimen for these patients would include an FQ (Mfx, or high-dose Lfx), a second-line injectable drug (at least until cultures become negative), and 2 drugs selected from Group 4 of this guideline. Z should also be included in this regimen if susceptible, due to its possible activity, taking into account that the susceptibility test is not reliable (conditional recommendation, very low [
] quality of evidence). The options listed in Table 5 should be considered.Recommended Basic Regimens for Patients With MDR-TB.
| MDR-TB cases, but without resistance to second-line drugs |
| Short regimen: 4a (Cm/Am+Mfxb+Pth/Eth+Cfz+E+Z+Hc)/5 (Mfxb+Cfz+E+Z) |
| Standard regimen: |
| Intensive phase (Cmd+Mfx/Lfx+Z+2 Group 4 drugse)/continuation phase (Mfx/Lfx+Z+2 Group 4 drugse) |
| MDR-TB cases with additional resistance to the FQ, SLID, both, or even broader XDR-TB resistance patterns |
| Consult experts and design a regimen that follows all the recommendations made in this guideline, looking for a minimum of 4 new drugs, following the rational classification provided (Groups 1 to 5), with the aim of including the maximum number of bactericidal and sterilizing drugs |
CP: continuation phase. Until completion of 21 months of treatment; IP: intensive phase. Until cultures become negative, or up to 6 months in case of extensive lesions. Continue for at least 4 months if the patient is not expectorating and no follow-up sputum tests can be performed.
Sources: Caminero et al.6; World Health Organization8; Caminero.55
The total duration of this combination of 4 new drugs plus Z is much more controversial. The conventionally recommended regimens of more than 21 months3,4,8,9 have not achieved success rates greater than 55%–70%, particularly due to high drop-out rates,40,57,58 which are clearly associated with the extensive length of treatment, and also with poor tolerance and toxicity. For this reason, this guideline recommends that priority be given to the 9- to 12-month schedule recently recommended by the WHO8 for all patients with R-resistant TB, or with MDR-TB who have not previously received FQ or SLID, or who show susceptibility in vitro to these 2 classes of antibiotics (conditional recommendation, very low [
] quality of evidence). This regimen consists of an initial phase of 4 months (or until sputum smears become negative) with kanamycin (Am or Cm in Spain), high-dose moxifloxacin, clofazimine, ethionamide/prothionamide, pyrazinamide, ethambutol, and high-dose H. The continuation phase will be of 5 months of high-dose moxifloxacin, clofazimine, ethambutol, and pyrazinamide. Patients must be closely monitored for possible adverse effects, primarily possible prolongation of the QTc interval on electrocardiogram, due in particular to the high moxifloxacin doses recommended in this regimen.Treatment of Patients With Multi-Drug Resistant Tuberculosis and Added Resistance to Fluoroquinolones, Second-Line Injectable Drugs, or Both, or Even Broader Patterns of ResistanceThe clinical and operational management of these forms of TB are already pose considerable difficulties.5,6 They must be treated by experts in the disease, in units that can guarantee close follow-up of treatment and appropriate management of adverse reactions. Possible regimens that can be administered to the vast majority of these patients are shown in Table 5.
Role of SurgerySurgery can also contribute to the success of the treatment of MDR-TB,6,8,59 although it can only be considered in the few patients that meet the following 3 conditions6: (1) reasonably localized resectable lesion; (2) sufficient respiratory reserve for the patient to tolerate surgery and the postoperative period; and (3) lack of medications available to design a curative regimen for the patient.6 If the last premise is met, surgery may be considered in patients with FQ-resistant MDR-TB or an even broader resistance pattern (conditional recommendation, very low [
] quality of evidence).Monitoring During Treatment and Evaluation of ResultsPatients should be monitored at least once a month during the intensive treatment phase, and then every 1 or 2 months during the continuation phase. Aspects that should be evaluated in each of these controls are listed in Table 6, and criteria for evaluating treatment outcomes are described in detail in Table 7.60
Follow-Up Required During TB Treatment.
| Control | M0 | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | +M12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medical visit | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Sputum smear (×2–3) | X | X | X | X | X | Xa | X | Xa | X | Xa | X | Xa | X |
| Culture (×2–3) | X | X | X | X | X | Xa | X | Xa | X | Xa | X | Xa | X |
| Clinical laboratory tests | X | X | X | X | X | X | X | X | X | X | X | X | X |
| EKG | X | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb |
| ENT audiometry | X | Xc | Xc | Xc | Xc | Xc | Xc | Xc | Xc | Xc | Xc | Xc | Xc |
| Chest radiograph | X | X | X | X | X | ||||||||
| Ophthalmological examination | X | Xd | Xd | Xd | Xd | Xd | Xd | ||||||
| Psychiatric evaluation | Xe | Xe | Xe | Xe | Xe |
Criteria for Evaluating Treatment Outcomes in TB Patients, Following WHO Recommendations.
| Result | Definition |
|---|---|
| Treatment outcomes in TB patients without rifampicin resistance and without MDR-TBa | |
| Cured | Patient with bacteriologically confirmed TB at the start of treatment and negative sputum smear or culture in the last month of treatment and on at least 1 previous test |
| Completed treatment | TB patient who completed treatment without evidence of failure, but without evidence of negative sputum smear or culture in the last month of treatment and on at least 1 previous test, either because the tests were not performed, or because the results are not available |
| Treatment failure | Patient with TB with positive sputum smear or culture in month 5 or later during treatment |
| Died | TB patient who dies for any reason before starting or during the course of treatment |
| Lost-to-follow-up | TB patient who did not start treatment or suspended treatment for 30 consecutive days |
| Not evaluated | TB patient without assigned treatment outcome. Includes cases transferred to another treatment unit, and cases in whom treatment outcome is unknown. |
| Outcomes of TB patients with rifampicin-resistant disease, or MDR/XDR-TB treated with second-line drugs | |
| Cured | Treatment completed as recommended by national guidelines without evidence of failure and 3 or more consecutive negative cultures at least 30 days apart, after the intensive phase |
| Completed treatment | Treatment completed as recommended by the national policy without evidence of failure, but without evidence of 3 or more consecutive negative cultures at least 30 days apart, after the intensive phase |
| Failed treatment | Treatment was discontinued or a permanent change of regimen or at least 2 anti-TB drugs were required due to: Failure to convertb at the end of the intensive phase, or Bacteriological reversionc in the continuation phase after conversion to a negative status, or Evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs, or Adverse drug reactions |
| Died | TB patient who dies for any reason before starting or during the course of treatment |
| Lost-to-follow-up | TB patient who did not start treatment or suspended treatment for 30 consecutive days |
| Not evaluated | TB patient without assigned treatment outcome. Includes cases transferred to another treatment unit, and cases in whom treatment outcome is unknown. |
Source: World Health Organization.60
Available evidence suggests that if a contact of a case with MDR-TB develops TB, they should receive the same treatment as the index case while pending susceptibility test results, with possible subsequent adjustment after the test results are received. If the secondary case is not confirmed microbiologically, as can happen in children, in paucibacillary TB, or in extrapulmonary TB, the regimen of the index case must continue. Systematic treatment of the tubercular infection in contacts of MDR-TB patients is not recommended, unless they have another risk factor for MDR-TB.3,4,6,61
ConclusionsAlthough TB resistances complicate treatment and the chances of success, following basic rules of management will ensure acceptable cure rates in the vast majority of patients. The basic principles and a summary of most of these guidelines can be found in Table 8.In all cases, an expert should be consulted when designing a treatment scheme for these patients. For this purpose, the foundation of national expert groups sponsored by the health authorities and/or the scientific societies is recommended.The first priority is still to offer patients with susceptible TB the appropriate treatment, in order to avoid the appearance of resistance.
Summary of Good Practice in the Management of MDR-TB.
| Steps | Considerations |
|---|---|
| 1. Diagnosis | Take in account: Drug history: 1 month of monotherapy, or the addition of a single drug to an ineffective treatment regimen is an important indicator of possible resistance to that drug, or possibly lower efficacy. Drug susceptibility testing (DST): very reliable for R and H; quite reliable for second-line injectable drugs and FQ; less reliable for S, E, and Z; unreliable for Eth/Pth, Cs, and PAS. The exact method and credibility remain to be determined for Lzd, Bdq Cfz, Dlm, and carbapenems HIV testing |
| 2. Number of drugs | At least 4 effective medications: never used in the past or with susceptibility demonstrated on DST, taking into account DST reliability mentioned in point 1 and possible cross-resistance At least 2 core drugs (at least 1 with high bactericidal activity and at least 1 other with sterilizing capacity) and 2 companion drugs to protect the core drugs |
| 3. Selection of drugs | Rational introduction according to Table 3 For MDR-TB, try to use first-line drugs if they are still effective. However, in this case, do not count them among the “4 effective drugs” High-dose levofloxacin or moxifloxacin A second-line injectable drug or S, if it is still susceptible and has not been used previously Use Group 4 drugs until 4 effective drugs have been included Consider high-dose H |
| 4. Treatment duration | Short regimen: 9 months. Intensive phase of at least 4 months or until sputum smear testing becomes negative. Continuation phase: 5 months Standard regimen: 21 months. Intensive phase: at least until sputum smear and culture become negative; always for at least 6 months. Even longer if there are less than 3 effective drugs in the continuation phase, or resistance to FQ is suspected. Continuation phase: at least until 21 months of treatment in total and 12 months with negative cultures Always with directly observed treatment |
| 5. Surgery | Consider only if the following 3 conditions are met: (1) less than 4 effective drugs; (2) localized lesions; and (3) sufficient respiratory reserve after resection In XDR and pre-XDR-TB especially, assess for resistance to FQ |
| 6. Ideal regimen | Standard: if only standard regimens have been administered in the past, both for first- and second-line treatment. Individualized: if second-line drugs other than standard treatments have been used, or in contacts of MDR patients who have used them. In the latter case, treat with the regimen that was effective in the index case |
In all cases, an expert should be consulted when designing a treatment scheme for these patients.
Adapted from: Caminero and Scardigli.38
The authors state that they have no conflict of interest.
Please cite this article as: Caminero JA, Cayla JA, García-García J-M, García-Pérez FJ, Palacios JJ, Ruiz-Manzano J. Diagnóstico y tratamiento de la tuberculosis con resistencia a fármacos. Arch Bronconeumol. 2017;53:501–509.