The diagnosis of peripheral pulmonary lesions (PPLs) is a challenging task for pulmonologists. Radial probe endobronchial ultrasound (R-EBUS) has been developed to enhance diagnostic yield. The objective of this study was to evaluate the effectiveness of R-EBUS in the diagnosis of PPLs.
MethodsA retrospective study was conducted on 174 patients diagnosed with PPLs who underwent EBUS-guided bronchoscopy. Histological examination of specimens obtained by transbronchial lung biopsy (TBLB) and cytological examinations of brushing smear, brush rinse fluid and bronchoalveolar lavage fluid (BALF) were evaluated for the diagnosis.
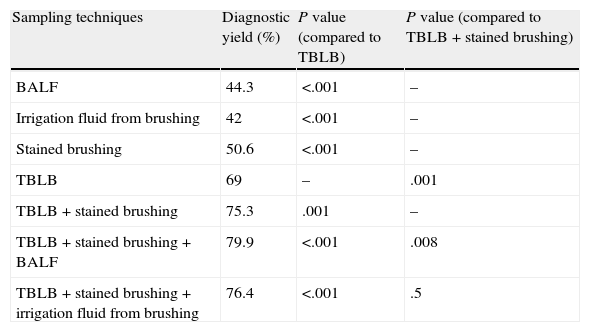
ResultsThe mean diameter of the PPLs was 25.1±10.7mm. The final diagnoses included 129 malignancies and 45 benign lesions. The overall diagnostic yield of EBUS-guided bronchoscopy was 79.9%. Neither size nor etiology of the PPLs influenced the diagnostic performance of EBUS-guided bronchoscopy (82.9% vs 74.6% for PPLs >20mm and PPLs ≤20mm; P=.19, and 82.9% vs 71.1% for malignancy and benign diseases; P=.09). TBLB rendered the highest yield among these specimens (69.0%, 50.6%, 42.0%, and 44.3% for TBLB, brushing smear, brush rinse fluid, and BALF, respectively; P<.001). The combination of TBLB, brush smear, and BALF provided the greatest diagnostic yield, while brush rinse fluid did not add benefits to the outcomes.
ConclusionR-EBUS-guided bronchoscopy is a useful technique in the diagnosis of PPLs. To achieve the highest diagnostic performance, TBLB, brushing smear and bronchoalveolar lavage should be performed together.
El diagnóstico de las lesiones pulmonares periféricas (LPP) constituye un verdadero reto para los neumólogos. La ecografía endobronquial radial (R-EBUS) es una técnica que se ha desarrollado para mejorar el rendimiento diagnóstico. El objetivo de este estudio fue evaluar la efectividad de la R-EBUS en el diagnóstico de las LPP.
MétodosSe llevó a cabo un estudio retrospectivo en 174 pacientes a los que se habían diagnosticado LPP y a los que se practicó una broncoscopia bajo guía de EBUS. Para el diagnóstico se evaluó el examen histológico de las muestras obtenidas mediante biopsia pulmonar transbronquial (BPTB) y los exámenes citológicos del frotis de cepillado, líquido de irrigación del cepillado y líquido de lavado broncoalveolar (LLBA).
ResultadosEl diámetro medio de las LPP fue de 25,1±10,7mm. Los diagnósticos finales fueron 129 en fermedades malignas y 45 lesiones benignas. El rendimiento diagnóstico global de la broncoscopia bajo guía de EBUS fue del 79,9%. Ni el tamaño ni la etiología de las LPP mostraron influencia alguna en el rendimiento diagnóstico de la broncoscopia bajo guía de EBUS (82,9% frente al 74,6% para las LPP de tamaño >20mm y las LPP de tamaño ≤20mm; p=0,19, y 82,9% frente al 71,1% para las enfermedades malignas y benignas; p=0,09). La BPTB fue la que obtuvo el máximo rendimiento de entre estas diversas muestras (69,0, 50,6, 42,0 y 44,3% para las muestras de BPTB, frotis de cepillado, líquido de irrigación de cepillado y LLBA, respectivamente; p<0,001). La combinación de BPTB, frotis de cepillado y LLBA aportó el máximo rendimiento diagnóstico, mientras que el líquido de irrigación de cepillado no añadió una mejora adicional de los resultados.
ConclusiónLa broncoscopia bajo guía de R-EBUS es una técnica útil en el diagnóstico de las LPP. Para alcanzar el máximo rendimiento diagnóstico deben utilizarse conjuntamente la BPTB, el frotis de cepillado y el lavado broncoalveolar.
Fluoroscopy-guided fiberoptic bronchoscopy (FB) has been used since the 1970s and has become standard practice in the diagnosis of peripheral pulmonary lesions (PPLs).1,2 The sampling instruments, brush or biopsy forceps, are inserted into the appropriate bronchus, and their position above the target is confirmed before obtaining samples under fluoroscopic control. Unfortunately, this bronchoscopic approach has limitations for evaluating small PPLs, and has a very low diagnostic yield for PPLs <2cm, ranging between 1% and 42%.3,4
Radial endobronchial ultrasound (R-EBUS) was developed in the 1990s to improve the diagnostic yield in PPLs. This technique has since been widely accepted by interventional pulmonologists given its excellent diagnostic performance for small, particularly <20mm, PPLs.5 The advantages of R-EBUS compared to fluoroscopy are the identification of anatomical sites and also the information it provides on the internal structure of the PPLs. Using R-EBUS, therefore, bronchoscopists can obtain a suitable sample of tissue from inside the lesion. In a meta-analysis of 16 studies published between 2002 and 2009, the combined diagnostic yield in peripheral lung cancers was 73%.6
Bronchoscopy-guided R-EBUS for the diagnosis of PPLs was first used in our center in June 2009. The aim of this study was to retrospectively evaluate the effectiveness of R-EBUS in the diagnosis of PPLs. The secondary objective was to define which bronchoscopic sampling technique gave the highest diagnostic yield in order to reduce the cost of samples obtained.
Material and MethodsWe conducted a retrospective review of 174 patients diagnosed with PPL and examined by R-EBUS-guided bronchoscopy between June 2009 and May 2013 in Ramathibodi Hospital, a university tertiary referral center in Bangkok (Thailand). This retrospective study was approved by the Ethics Committee for Experimentation in Humans of Ramathibodi Hospital, Faculty of Medicine, Mahidol University. PPL was defined as a pulmonary lesion surrounded by lung parenchyma and not endoscopically visible by bronchoscopy. A computed tomography (CT) scan was performed in all cases, and a subsegmental bronchus corresponding to the PPL was chosen prior to the bronchoscopic procedure. The size of the lesion was recorded by its maximum diameter as seen in CT scan. Written informed consent was obtained of all patients before the bronchoscopic procedure.
FB-bronchoscopy (BF-P180, Olympus, Tokyo: outer diameter, 4.9mm; channel diameter, 2.0mm) was performed transnasally with local anesthesia. The whole bronchial tree was bronchoscopically examined to the level of the subsegmental bronchi. Next, an R-probe EBUS with guide sheath (GS) (UM-S20-17S, Olympus, Tokyo, Japan) was inserted through the FB working channel and was advanced to the targeted subsegmental bronchus. EBUS and fluoroscopy images were examined to confirm that the probe and the GS had reached the targeted lesions. The R-EBUS probe was then moved within the lesion to examine the most suspicious areas, in order to obtain tissue specimens with fluoroscopic control.7 Once the appropriate site was found, the probe was removed under fluoroscopy, leaving the GS in place. A biopsy forceps and a bronchial brush were introduced through the GS to obtain samples in the site marked by fluoroscopy. In general, five brushing and biopsy sampling attempts were performed.8 Finally, the GS was removed and bronchoalveolar lavage was performed by instilling 50ml of normal saline followed by aspiration. Instillation of 50ml of normal saline was repeated to a total of 100–150ml in order to collect at least 50ml of fluid, which was considered adequate lavage.
The samples obtained by transbronchial lung biopsy (TBLB) were gathered in a microtube filled with 0.5ml of normal saline. After TBLB completion, 1ml of 10% formalin was added and the samples were centrifuged at 6000rpm for 5min. The pellet was processed as a cell block for pathologic evaluation. Bronchial brushing samples were smeared on a microscopy slide and fixed immediately in a flask containing 95% alcohol, followed by staining using the Papanicolaou method. Bronchial brushing was irrigated with 3ml of sterile normal saline to obtain new samples. In addition, 1ml of sterile normal saline solution was used to irrigate the material left in the GS, washing it to the brushing fluid. This fluid was sent to the laboratory for centrifugation and staining using the Papanicolaou method. The bronchoalveolar lavage fluid (BALF) was processed for cytology, Gram, acid-fast bacilli and modified Giemsa staining, and microbial culture.
Statistical AnalysisContinuous variables are expressed as mean±standard deviation (SD) and categorical variables are presented as percentages. To explore the association between the independent variables and the diagnostic yield, bilateral Student's t-test was used for continuous variables and discrete variables were analyzed with the χ2 test. The diagnostic yield of each of the sampling techniques was compared with the Cochran Q test. If statistical significance was found, McNemar test was used to compare the various techniques. All statistical tests were two-sided, and a P-value <.05 was considered statistically significant. All data were analyzed using SPSS v16.0 for Windows (SPSS Inc., Chicago, IL, USA).
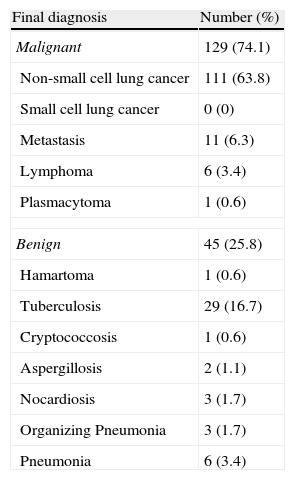
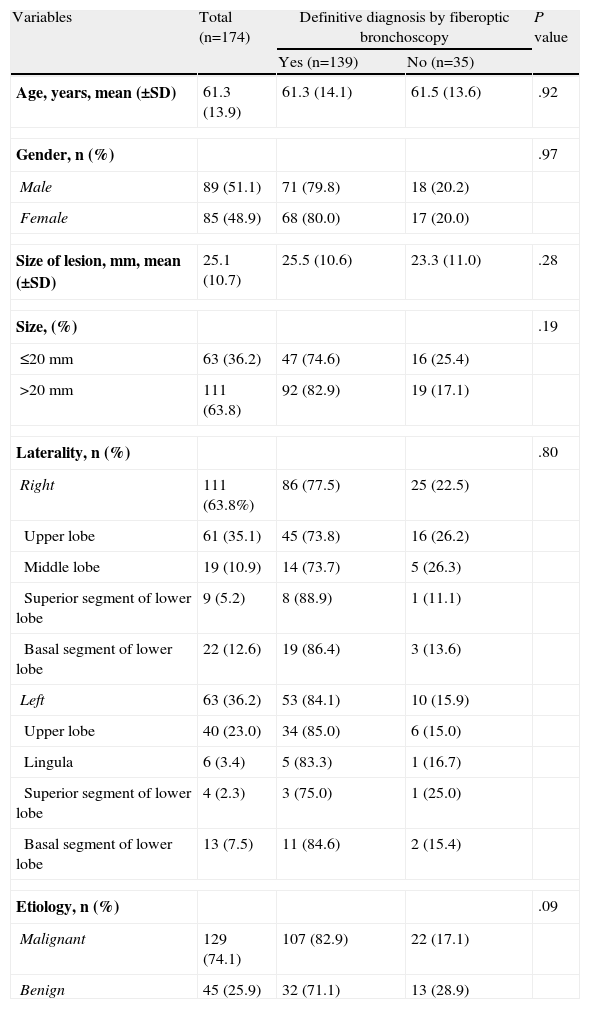
ResultsA total of 174 patients (89 men and 85 women) were retrospectively evaluated. The final diagnoses are shown in Table 1. Mean (±SD) age was 61.3±13.9 years. Mean (±SD) diameter of PPL was 25.1±10.7mm. The overall diagnostic yield of EBUS-guided bronchoscopy was 79.9%. The baseline patient characteristics and factors related to the diagnostic yield of R-EBUS-guided FB are summarized in Table 2. Neither the size nor the etiology of PPL influenced the diagnostic yield of EBUS-guided FB.
Final Diagnosis of Peripheral Lung Lesions in 174 Patients.
| Final diagnosis | Number (%) |
| Malignant | 129 (74.1) |
| Non-small cell lung cancer | 111 (63.8) |
| Small cell lung cancer | 0 (0) |
| Metastasis | 11 (6.3) |
| Lymphoma | 6 (3.4) |
| Plasmacytoma | 1 (0.6) |
| Benign | 45 (25.8) |
| Hamartoma | 1 (0.6) |
| Tuberculosis | 29 (16.7) |
| Cryptococcosis | 1 (0.6) |
| Aspergillosis | 2 (1.1) |
| Nocardiosis | 3 (1.7) |
| Organizing Pneumonia | 3 (1.7) |
| Pneumonia | 6 (3.4) |
Baseline Characteristics and Influence on the Diagnostic Yield of Bronchoscopy.
| Variables | Total (n=174) | Definitive diagnosis by fiberoptic bronchoscopy | P value | |
| Yes (n=139) | No (n=35) | |||
| Age, years, mean (±SD) | 61.3 (13.9) | 61.3 (14.1) | 61.5 (13.6) | .92 |
| Gender, n (%) | .97 | |||
| Male | 89 (51.1) | 71 (79.8) | 18 (20.2) | |
| Female | 85 (48.9) | 68 (80.0) | 17 (20.0) | |
| Size of lesion, mm, mean (±SD) | 25.1 (10.7) | 25.5 (10.6) | 23.3 (11.0) | .28 |
| Size, (%) | .19 | |||
| ≤20mm | 63 (36.2) | 47 (74.6) | 16 (25.4) | |
| >20mm | 111 (63.8) | 92 (82.9) | 19 (17.1) | |
| Laterality, n (%) | .80 | |||
| Right | 111 (63.8%) | 86 (77.5) | 25 (22.5) | |
| Upper lobe | 61 (35.1) | 45 (73.8) | 16 (26.2) | |
| Middle lobe | 19 (10.9) | 14 (73.7) | 5 (26.3) | |
| Superior segment of lower lobe | 9 (5.2) | 8 (88.9) | 1 (11.1) | |
| Basal segment of lower lobe | 22 (12.6) | 19 (86.4) | 3 (13.6) | |
| Left | 63 (36.2) | 53 (84.1) | 10 (15.9) | |
| Upper lobe | 40 (23.0) | 34 (85.0) | 6 (15.0) | |
| Lingula | 6 (3.4) | 5 (83.3) | 1 (16.7) | |
| Superior segment of lower lobe | 4 (2.3) | 3 (75.0) | 1 (25.0) | |
| Basal segment of lower lobe | 13 (7.5) | 11 (84.6) | 2 (15.4) | |
| Etiology, n (%) | .09 | |||
| Malignant | 129 (74.1) | 107 (82.9) | 22 (17.1) | |
| Benign | 45 (25.9) | 32 (71.1) | 13 (28.9) | |
Table 3 shows the diagnostic yield of several bronchoscopy sampling techniques. Of these, TBLB gave the highest diagnostic yield (P<.001, Cochran Q test). Combined TBLB and brushing gave a higher yield than TBLB alone (P=.001). In addition, the combination of BALF with TBLB and brushing gave the maximum diagnostic yield (P=.008), while the brushing irrigation fluid did not improve the clinical results obtained with this combination (P=.5).
Diagnostic Yield of Different Sampling Techniques by Bronchoscopy.
| Sampling techniques | Diagnostic yield (%) | P value (compared to TBLB) | P value (compared to TBLB+stained brushing) |
| BALF | 44.3 | <.001 | – |
| Irrigation fluid from brushing | 42 | <.001 | – |
| Stained brushing | 50.6 | <.001 | – |
| TBLB | 69 | – | .001 |
| TBLB+stained brushing | 75.3 | .001 | – |
| TBLB+stained brushing+BALF | 79.9 | <.001 | .008 |
| TBLB+stained brushing+irrigation fluid from brushing | 76.4 | <.001 | .5 |
TBLB: transbronchial lung biopsy; BALF: bronchoalveolar lavage fluid.
In our study, none of the patients had major bleeding requiring intervention. In addition, no significant adverse effects such as pneumothorax or respiratory distress were observed over the study period.
DiscussionThe diagnostic yield of R-EBUS in PPLs varied widely, with values ranging from 46% to 84%.9 When initially introduced, it was thought that combining R-EBUS with fluoroscopy would be unnecessary, given concerns about exposure to radiation. However, the diagnostic yield of R-EBUS alone was lower than that of R-EBUS with fluoroscopy.6 This is due to the movement of PPLs during breathing, the shifting of the GS away from the examination target, and the extension or retraction of the GS after examination and serial sampling.10,11 At present, the combination of R-EBUS with fluoroscopy is widely accepted as a standard procedure.
Kurimoto et al.5 introduced a GS specifically for R-EBUS in 2004. It has a probe on the tip of the GS that is visible by fluoroscopy, providing confirmation that the GS remains in position after withdrawal of the R-EBUS. The advantages of GS include repeated access to the lesion for sampling purposes, avoiding the collapse of the small bronchial branches after specimens are obtained, and the possibility of applying a wedge in the lesion to prevent bleeding from the biopsy site spreading to the proximal bronchus.
The characteristics of PPLs also affect the diagnostic yield of R-EBUS-guided FB. Many studies have reported that lesion size significantly affects diagnostic yield of R-EBUS.5,6,12–14 In our study, although the diagnostic yield for PPLs >20mm was greater than that from <20mm lesions, the difference was not statistically significant. One possible reason for this may be that a bronchus sign on CT can indicate the position of the probe in relation to the lesion. Lesion size was a significant factor in predicting PPL visibility by R-EBUS.14 Huang et al.14 found that visibility for PPLs <20mm was only 48.6%. Results from lesions that were not visible by R-EBUS were not diagnostic. In our retrospective study, the data were obtained in standard clinical practice, and this may cause unavoidable bias in the selection of the diagnostic approach for PPLs. If the bronchus leading to the PPL could not be identified on CT, other diagnostic approaches such as transthoracic aspiration were used. Only cases where bronchi leading to lesions could be identified, irrespective of their size, were selected for R-EBUS-guided FB, so visibility by R-EBUS was possible in most cases. Consequently, lesion size was not statistically significant in our series. The same was seen by Yamada et al.,8 who suggested, after multivariate analysis, that only the position of the probe in relation to the lesion, and not its size, predicted diagnostic yield. Our diagnostic yield was 79.9%, comparable to that found in patients with positive R-EBUS images in other studies.8,10,13,14
Another factor significantly influencing diagnostic yield was etiology.5,12,14 Although the diagnostic yield from malignant lesions in this study was higher than that obtained from benign disease, differences were not statistically significant. This difference in results was probably due to the low prevalence of benign lesions and the small number of patients.
The sampling technique is another cause for concern with regard to the diagnostic yield of FB. TBLB has been shown to be the most effective technique for obtaining diagnosis,5,8 and at least 5 biopsy specimens are required.8
Specimens obtained using GS biopsy forceps are larger than those obtained from standard biopsies. Thus, all the biopsy specimens obtained from each patient were gathered in the micropipette tube and cell blocks were prepared for histological examination of the tissue. The advantages of cell blocks compared to conventional pathology preparations have not been investigated and should be explored in the future.
Combination with other techniques for obtaining diagnostic specimens and TBLB specimens may increase diagnostic yield. We found that adding BALF diagnostic results to the combination of TBLB and brushings can raise total diagnostic yield, and this combination produced the maximum diagnostic results. In contrast, brushing rinse fluid obtained from bronchial brushing and the GS prepared in a smear for additional cytological examination did not increase diagnostic yield. This latter technique can thus be ruled out, since the costs involved are disproportionate to utility.
In conclusion, we have shown that R-EBUS-guided FB is a useful technique in the diagnosis of PPLs. With regard to techniques for obtaining specimens, the combined use of TBLB, brushing and bronchoalveolar lavage should be used to obtain the maximum diagnostic yield.
Ethical ConsiderationsThis retrospective study protocol was approved by the Ethics Committee for Experimentation in Humans of Ramathibodi Hospital, Faculty of Medicine, Mahidol University.
FundingNone.
AuhorshipViboon Boonsarngsuk designed and conducted the study, performed the R-EBUS-guided bronchoscopy, analyzed the data, prepared and revised the manuscript.
Wasana Kanoksil processed and analyzed cytology and histology samples.
Sarangrat Laungdamerongchai conducted the study, and collaborated in the R-EBUS-guided bronchoscopies.
Conflicts of InterestThe authors declare no conflict of interest.
The authors thank Dr. Pensupa Raweelert for constructive suggestions and English revision.
Please cite this article as: Boonsarngsuk V, Kanoksil W, Laungdamerongchai S. Diagnóstico de lesiones pulmonares periféricas con broncoscopia bajo guía de ecografía endobronquial radial. Arch Bronconeumol. 2014;50:379–383.