In recent years, there has been debate regarding the diagnostic accuracy of computed tomography (CT) in the identification of lung metastases and the need for lung palpation to determine the number of metastatic nodules. The aim of this study was to determine in which patients the CT scan was more effective in detecting all metastases.
MethodsWe studied all patients who underwent curative thoracotomy for pulmonary metastasis between 1998 and 2012. All cases were reviewed by two expert pulmonary radiologists before surgery. Statistical analyses were performed using Systat version 13.
ResultsThe study included 183 patients (63.6% male) with a mean age of 61.7 years who underwent 217 interventions. The CT scan was correct in 185 cases (85.3%). Discrepancies observed: 26 patients (11.9%) with more metastases resected than observed and 6 cases (2.8%) with fewer metastases. In patients with one or two metastases of colorectal origin or a single metastasis of any other origin, the probability of finding extra nodules was 9.5%. In the remaining patients, the probability was 27.8%, with statistically significant differences (P=.001). The mean age of the patients in whom no unobserved nodules were detected was 62.9 years compared to 56.5 years on average in patients who were free from any metastases (P=.001).
ConclusionsPatients older than 60 years, with one or two metastases of colorectal origin or a single metastasis from any other origin were considered to be the group with low probability of having more metastases resected than observed.
En los últimos años existe un debate en relación con la exactitud diagnóstica de la tomografía axial computarizada (TAC) para identificar metástasis pulmonares y la necesidad de la palpación pulmonar para determinar el número de nódulos metastásicos. El objetivo del estudio fue determinar en qué pacientes era más eficaz la TAC para detectar todas las metástasis.
MétodosSe estudiaron todos los pacientes operados de metástasis pulmonar con intención curativa a través de toracotomía entre 1998 y 2012. Todos los casos fueron revisados preoperatoriamente por 2radiólogos expertos en pulmón. Para el análisis estadístico se utilizó el programa Systat versión 13.
ResultadosCiento ochenta y tres pacientes (63,6% varones) con una edad media de 61,7años a los que se les realizaron 217 intervenciones. La TAC acertó en 185 casos (85,3%). Discordancias observadas: 26 pacientes (11,9%) con más metástasis resecadas que las observadas y 6 casos (2,8%) con menos metástasis. Agrupando a los pacientes de origen colorrectal con una o 2 metástasis y metástasis única de cualquier origen, la probabilidad de resecar nódulos extras fue del 9,5%. En el resto la probabilidad fue del 27,8%, observándose diferencias estadísticamente significativas (p=0,001). La edad media de los pacientes en los que no aparecieron nódulos no observados fue de 62,9años, frente a 56,5años de media en los pacientes que se escapaba alguna metástasis (p=0,001).
ConclusionesSe consideró grupo con baja probabilidad de resecar más metástasis que las observadas a los pacientes mayores de 60años con una o 2metástasis de origen colorrectal o una de otro origen.
In recent years, the resection of pulmonary metastases from primary extrapulmonary neoplasia has become an essential part of the activity of thoracic surgery departments.1 Evidence from case series suggests that complete excision in selected patients results in greater survival than non-surgical treatments.1 The goal in the treatment of pulmonary metastases is complete removal of the metastasis with minimal loss of lung parenchyma.1,2 Chest computed axial tomography (CT) is considered the test of choice before performing metastasectomy for determining the extension of the intrathoracic lesion.3 In recent years, there has been a debate in relation to the diagnostic accuracy of this procedure, and the need for lung palpation to determine the number of metastatic nodules present in the lung.4,5 A true reflection of the controversial nature of the subject is that in the book Difficult decisions in thoracic surgery: An evidence based approach, two chapters are dedicated to pulmonary metastasectomy,6,7 with the authors concluding that:
- -
In relation to the need for palpation of both lungs,6 the absence of data showing improved survival after palpation of contralateral undetected nodules justifies unilateral access for nodules detected unilaterally.6
- -
Comparing the videothorascopic (VATS) approach with thoracotomy7: (a) thoracotomy allows additional nodules to be identified in 20% of cases; (b) incomplete resection is considered therapeutic failure, but incomplete resection is not synonymous with the presence of a radiologically undetectable lesion post-VATS; (c) there is no scientific evidence to support that thoracotomy provides clinical improvement compared to VATS in terms of survival, and (d) the claim that thoracotomy has more advantages than VATS is supported solely by expert opinion. The available literature suggests that they are equivalent, and both approaches seem to be appropriate for resection of a solitary pulmonary metastasis.
Determining the best form of approach is still under debate at present. In contrast to studies in which up to 20% of metastases not identified by CT scan were detected, which would have been missed by CT and VATS,8 there is an increasing number of scientific studies in which comparable survival and disease-free survival have been observed in patients who have undergone VATS compared to those who have undergone thoracotomy.5,9,10 Gossot et al.9 in patients with osteosarcoma metastases and Nakajima et al.5 and Nakaset et al.11 in subjects with metastases of colorectal origin, showed comparable survival and disease-free survival in patients who had undergone surgery by VATS compared to those who had undergone surgery by thoracotomy. The improvement in imaging tests in recent years is resulting in an improvement in the precision of CT, as we can see in the article published in 2008 by Kang et al.,12 in which the accuracy of CT reached 96% in metastases of colorectal origin.
MethodsPatients who underwent surgery for pulmonary metastasis with curative intent between 1 January 1998 and 30 June 2012 were studied. During this period, 229 operations were carried out, 12 of which were performed using VATS, and therefore excluded from the study. Patients enrolled underwent thoracotomy and unilateral palpation to resect all the nodules palpated. The following were considered criteria for surgery: controlled primary tumour (or with a possibility of controlling it), absence of extrapulmonary metastases (except for resectable liver metastases), the patient was sufficiently healthy to tolerate the surgery (with good lung function tests) and with technically resectable metastases.
SurgeryAll patients were operated on by the Thoracic Surgery Department of Hospital Universitario Donostia under general anaesthetic, single lung ventilation and posterolateral thoracotomy. As part of the pre-operative study, all patients underwent lung function tests (spirometry and diffusion), fibrobronchoscopy and CT. PET-CT was also performed on all patients from 2006 onwards. The procedure followed for the surgery was always the same: (a) examination of the parietal and visceral pleura; (b) release of pleuropulmonary adhesions (if any); (c) identification of pulmonary nodule(s), and (d) when the nodule was peripheral: intra-operative biopsy with atypical resection. If the intra-operative biopsy confirmed metastasis, no more lung was resected. If the pathologist was unable to determine whether the nature of the lesion was metastatic or a primary lung tumour, lobectomy with lymphadenectomy was then performed. In cases in which the tumour was central and did not permit intraoperative biopsy, lobectomy was carried out directly. Lymphadenectomy was performed in patients with metastasis and suspected mediastinal lymph node involvement.
Preoperative StudyAll cases were reviewed preoperatively by 2 radiologists specialising in lung disease. A non-multislice one coronal helical CT (Siemens) with 5mm slices was performed on patients who underwent surgery before November 2002 and a non-multislice two coronal helical CT (Siemens Somatom Volume Access) with 5mm slices and contrast was performed on patients who underwent surgery after this date. All patients underwent surgery within 40 days of the CT scan.
Statistical AnalysisThe objective was to compare the number of metastases identified by CT with the number of metastases resected per patient (histologically confirmed) after manual palpation, so benign nodules palpated and resected during the surgery were not taken into account. Systat version 13 was used for the statistical analysis. Quantitative variables were described using the interval, median and mean, and the qualitative variables using absolute and relative frequencies in percentage. Differences between both groups were measured using Fisher's exact test or the Chi-squared test for categorical variables and the Mann–Whitney test for quantitative variables.
The study was approved by the Clinical Research Ethics Committee, as stated in Act no. 02/2012, having obtained informed consent from the patients.
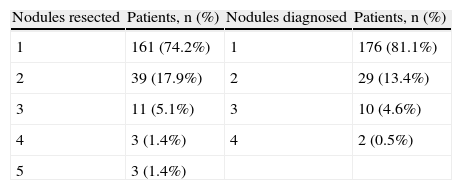
ResultsOne hundred and eighty-three (183) patients (63.6% males) were considered for the study, in whom 217 surgeries were carried out. The mean age was 61.7 years (median, 63; range, 24–82). Comparing the nodules detected in the CT scan with those resected in the surgery, the CT was accurate in 185 cases (85.3%). Among the discrepancies observed were: 26 patients (11.9%) with more metastases resected than observed and 6 cases (2.8%) with fewer metastases than observed. The main descriptive study variables are summarised in Table 1.
Main Descriptive Variables of the Study.
| Nodules resected | Patients, n (%) | Nodules diagnosed | Patients, n (%) |
| 1 | 161 (74.2%) | 1 | 176 (81.1%) |
| 2 | 39 (17.9%) | 2 | 29 (13.4%) |
| 3 | 11 (5.1%) | 3 | 10 (4.6%) |
| 4 | 3 (1.4%) | 4 | 2 (0.5%) |
| 5 | 3 (1.4%) |
| Origin | Patients, n (%) | Resection performed | Patients, n (%) |
| Colorectal | 123 (56.7%) | Atypical resection | 128 (58.9%) |
| Sarcoma | 29 (13.4%) | Lobectomy | 71 (32.7%) |
| Kidney | 14 (6.5%) | Bilobectomy | 1 (0.5%) |
| Bladder | 9 (4.1%) | Pneumonectomy | 6 (2.8%) |
| Breast | 7 (3.2%) | Lobectomy+atypical resection | 7 (3.2%) |
| Other | 35 (16.1%) | Other | 4 (1.8%) |
A total of 299 metastases were resected and their size analysed. Those diagnosed by CT had a mean size of 2.4cm (median, 2.0cm; range, 0.4–8cm) while those not observed had a mean size of 0.6cm (median, 0.5cm; range, 0.2–1cm). Statistically significant differences were observed when the two means were compared (P<.001). Tumours measuring less than 1cm were underdiagnosed in 19.4% of cases, tumours between 1 and 2cm in 12.8% and tumours over 2cm in 8.4%. The differences were not statistically significant (P=.213), although it was observed that the smaller the diameter, the higher the probability of not detecting any metastases.
Analysis According to the Number of Metastases Observed in the Computed Axial TomographyA single nodule was seen in 176 cases, of which the assessment was correct in 158 cases (89.8%); 2 nodules were resected in 16 patients (9.1%) and 3 nodules in 2 patients (1.7%).
Two nodules were found in 29 patients, with an accuracy of 72.4% (20 patients); there was only one metastatic tumour in 3 patients (10.3%), and in 5 patients (17.2%) more metastases were detected in the tissue specimen than were observed.
Three nodules were observed in 10 patients; in 5 patients the assessment was correct (50.0%); in 2 cases (20.0%) fewer were seen, and in 3 cases (30.0%) more nodules appeared.
Four nodules were seen on 2 occasions: 3 nodules were resected in the specimen in one (50.0%) and 4 nodules in the other (50.0%).
Comparing the accuracy of the CT when one metastasis was observed (89.8%) against the accuracy when it said that there were more (65.8%), there were statistically significant differences (P<.001). However, on comparing the number of patients in whom a metastasis was missed, it was observed that in patients in whom the CT said that there was one, more tumours were resected than observed in 10.0% of patients, compared to 19.0% (P=.099) when more than one was observed on the CT, which means that when the CT says that there is a single metastasis, it has a Cohen kappa of 0.72, which represents substantial agreement between the data observed by CT and resected after surgery.
Analysis According to HistologyThe most commonly found histology was intestinal adenocarcinoma (111 patients who underwent 123 procedures). Twenty-three surgeries were performed in patients with tumours of urogenital origin and 29 sarcomas. Metastases were underdiagnosed in 8.9% of colorectal cancers, 13.8% of sarcomas, 21.7% of urogenital tumours and in 14.3% of other origins. Comparing the different groups, although there was a greater tendency for metastases to be missed in the group of urogenital origin, no statistically significant differences were observed (P=.327).
Grouping patients of colorectal origin with one or 2 metastases and single metastasis of any origin, the probability of resecting additional nodules was 9.5%. Comparing this with the rest, where the probability was 27.8%, statistically significant differences were observed (P=.001).
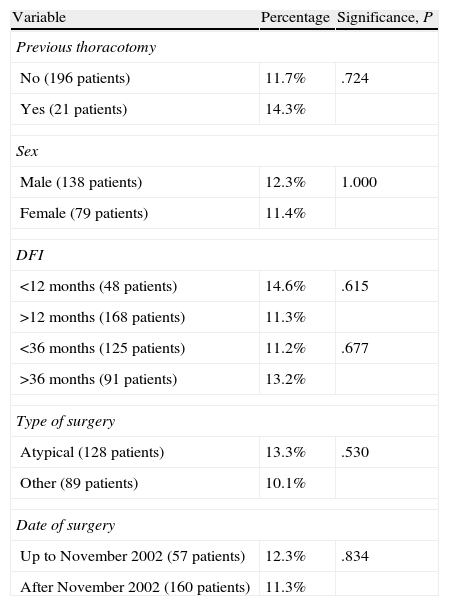
Other VariablesOther variables were analysed, such as age, observing that the mean age of patients in whom unobserved nodules did not appear was 62.9 years, compared to a mean of 56.5 years in patients in whom a metastasis was missed (P=.001). No statistically significant differences were observed in relation to the disease-free interval, performance of a previous thoracotomy, sex, date or type of surgery (Table 2).
Patients in Whom More Metastases Were Resected Than Observed by CT.
| Variable | Percentage | Significance, P |
| Previous thoracotomy | ||
| No (196 patients) | 11.7% | .724 |
| Yes (21 patients) | 14.3% | |
| Sex | ||
| Male (138 patients) | 12.3% | 1.000 |
| Female (79 patients) | 11.4% | |
| DFI | ||
| <12 months (48 patients) | 14.6% | .615 |
| >12 months (168 patients) | 11.3% | |
| <36 months (125 patients) | 11.2% | .677 |
| >36 months (91 patients) | 13.2% | |
| Type of surgery | ||
| Atypical (128 patients) | 13.3% | .530 |
| Other (89 patients) | 10.1% | |
| Date of surgery | ||
| Up to November 2002 (57 patients) | 12.3% | .834 |
| After November 2002 (160 patients) | 11.3% | |
DFI, disease free interval.
One coronal CT was used up to November 2012, after which a 2 coronal CT was used (both with 5mm slices).
Adding the variable of age, patients can be divided into 2 large groups. (1) Group with a low probability of detecting extra metastases: patients over 60 years of age, with one or 2 metastases of colorectal origin or one of any other origin, where metastasis was missed in only 4.4% of patients. (2) Group with a high probability of detecting extra metastases: patients under 60 years of age, with more than 2 metastases of colorectal origin or more than one of any other origin (20.4% of patients with more metastases resected than observed, P<.001).
DiscussionThe role of VATS for resecting pulmonary metastases is controversial,7,13,14 as the advantages of VATS are set against its major drawback: the inability to palpate the lung. Among the advantages are: excellent visualisation of the pleural surface,1 smaller immune response to surgical stress,15 reduction in post-operative pain,15 shorter hospital stay15 and post-operative recovery with fewer complications than in thoracotomy.10,16,17 Furthermore, since up to 50% of patients undergoing surgery for pulmonary metastases will present new pulmonary nodules,15 and since VATS causes fewer pleuropulmonary adhesions, it may favour repeat surgery in patients with recurrence.10,12,18 On the contrary, the importance of not being able to palpate the lung is that we can find numerous publications in which around 20% of previously undiagnosed metastases were detected.4,8,19–21
In our initial experience with VATS for pulmonary metastasis, we observed overall and disease-free survival comparable to that of patients who had undergone thoracotomy and the same rate of pulmonary recurrence.22 These results agree with the study published by Nakas et al.,11 who compared 25 patients who underwent thoracotomy with 27 VATS patients. They did not observe any statistically significant differences in survival, recurrence and recurrence in the same lung. Later, Nakajima et al.5 would reproduce the same results in patients with metastases of colorectal origin and Gossot et al.9 in patients with sarcomatous metastases. Therefore, we consider that VATS may be a therapeutic option in selected patients,23 and that to try to minimise the negative effect of the absence of lung palpation, it would be interesting to study the possibility of grouping patients by the probability of having nodules not detectable by CT.
According to results from the International Registry of Lung Metastases,24 the accuracy of CT for detecting the exact number of pulmonary nodules was 61%. With these findings, they concluded that manual bilateral palpation was necessary for the final staging and to be able to resect all the metastases.
In 2007, Parsons et al.19 published the results observed after operating on 60 patients with single lung metastases on CT; bilateral palpation was performed on all patients, finding 23% of patients with metastases in the supposedly healthy hemithorax. Thus they concluded, like the registry, that bilateral palpation was obligatory in metastasectomy. Similarly, Roth et al.25 compared the results of patients who had undergone sternotomy (which allows both lungs to be palpated with a single incision) with thoracotomy, where bilateral metastases not detected by CT were detected in patients who underwent sternotomy. However, on analysing the survival in both groups, no differences were observed when they were compared.25 Later, Younes et al.,26 in the same research line, determined that most patients with unilateral lung metastases detected by CT had unilateral disease, and that delaying the contralateral thoracotomy until the lesions became visible by CT did not affect survival.26 For these reasons, Patel and DeCamp6 concluded in their review that the absence of data showing improved survival after palpation of contralateral undetected nodules justifies unilateral access for nodules detected unilaterally.
In 2008, a survey of members of the European Society of Chest Surgery was published,27 in which 98% of surgeons surveyed answered that they treated only one hemithorax in cases of single lung involvement detected by CT. From the same survey,27 it appears palpation is considered necessary by 65% of surgeons, i.e. they consider it unacceptable that up to 20% of metastases could be missed if minimally invasive surgery is performed. For this reason, it is particularly interesting to define the group of patients in which CT is most accurate, as they would be ideal candidates for VATS.
In most studies to determine the accuracy of CT, nodules identified by CT were compared with those resected4,8,12,20,21; these latter could be either metastatic or benign (around 30%–40% of the extra nodules resected were benign8,19,20). To facilitate the statistical analysis in our study, only metastases not detected by CT were analysed, as the presence of non-resected benign nodules does not represent a decline in survival.
In our study, unobserved metastases were resected in 26 patients (11.9%). Parsons et al.19 found that 46% of patients had nodules that were not detected previously. Analysing the characteristics of the patients included in their series in detail, it is notable that most of the patients who underwent surgery had metastases of sarcomatous origin. Chun et al.,17 in contrast, after reviewing 120 patients with tumours of colorectal origin, detected unidentified metastases in 25% of patients. Cerfolio et al.28 and Kang et al.12 found differences when comparing patients with metastases of colorectal origin with those of sarcomatous origin: patients with sarcomas had more undetected metastases, and in the non-sarcomatous group, the CT obtained a negative predictive value of 96% and a sensitivity of 97%.12 Similarly, in the study by Chun et al.,17 the sensitivity of CT in patients with a single metastasis of colorectal origin was 95%.
The value of PET has been analysed to try to improve the pre-operative diagnosis. This can provide information on infiltration of mediastinal lymph nodes3 and for detecting extrapulmonary disease,29 but it does not aid in the detection of extrapulmonary nodules, since the sensitivity of PET for detecting nodules smaller than 10mm is 30%.29
According to the results obtained in our study, in the group with a low probability of detecting extra metastases (patient over 60 years of age with one or 2 metastases of colorectal origin or one of any other origin), metastasis was missed in only 4.4% of patients. These would be the ideal candidates for treatment using minimally invasive surgery, since they would benefit from its advantages, reducing as far as possible the harmful effect that not resecting all the metastases would have. On the contrary, a group with a high probability of detecting extra metastases would be patients under 60 years of age with more than 2 metastases of colorectal origin or more than one of any other origin (20.4% of patients with more metastases resected than observed). In deciding the procedure in this group, both surgeon and patient must discuss the pros and cons of each approach, knowing the advantages and limitations of each technique.
ConclusionsThe group considered to have a low probability of resecting more metastases than observed were patients older than 60 years with one or 2 metastases of colorectal origin or one of any other origin.
The group with a low probability of resecting more metastases than observed are ideal candidates for video thoracoscopic surgery. In the remaining patients, the probability that a metastasis would be missed and the benefits of VATS compared to thoracotomy would have to be assessed more carefully.
Study LimitationsThe main study limitation is that this is a retrospective study, so that the conclusions derived from it must be considered as a starting point for future prospective studies.
Authors’ ContributionsJZ: design, writing of the first draft and the final manuscript; BA: literature review and collaboration in writing the first draft; JI: data collection, review of the manuscript; MM: preoperative imaging study, review of the manuscript; FB: preoperative imaging study; MM: data collection; CL: pathology study; JE: statistical analysis, review of the manuscript.
FundingThis study did not receive funding of any type.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Zabaleta J, Aguinagalde B, Izquierdo JM, Mendoza M, Basterrechea F, Martin-Arruti M, et al. Determinación de grupo de bajo riesgo para tener nódulos metastásicos no detectados por tomografía axial computarizada en la cirugía de las metástasis pulmonares. Arch Bronconeumol. 2013;49:518–522.