Diffuse pulmonary ossification is a rare condition that is characterized by heterotopic ossification (HO) of the lung parenchyma. The lack of specific symptoms and the possibility of underestimating computed tomography (CT) findings result in a post-mortem diagnosis in the majority of cases. Two forms of diffuse ossification have been described: nodular and dendritic. Nodular pulmonary ossification has been explained by cellular stagnation and organization after congestive processes, such as in cardiac valvular disorders, leading to calcified or ossified mass formation within the alveolar spaces. Dendriform pulmonary ossification (DPO) originates within the alveolar septa and spreads into the alveolar spaces in the so-called dendritic pattern. It usually contains fat or marrow elements, and has been mainly described in patients with chronic obstructive pulmonary diseases and some fibrotic interstitial lung diseases (ILD), as a non-specific sign of advanced stage disease.1
Here we report the case of a patient with subclinical familial pulmonary fibrosis (FPF) identified during the screening program for first-degree relatives of idiopathic pulmonary fibrosis (IPF) cases with telomere shortness, and who was finally diagnosed after a surgical lung biopsy that demonstrated DPO and usual interstitial pneumonia (UIP) pattern.
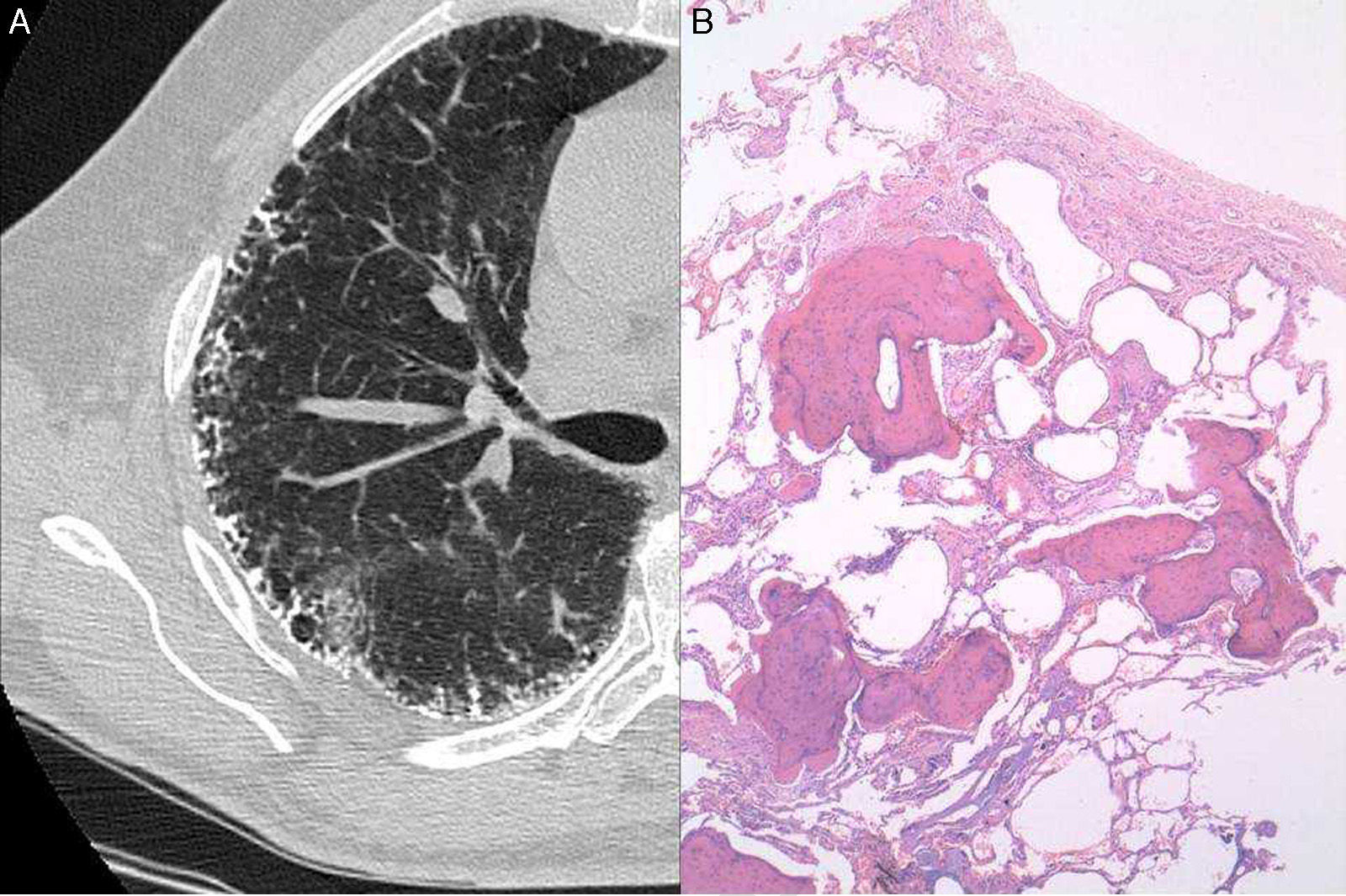
A 63-year-old man with a history of mild cigarette smoking and no other relevant conditions was under study in our Unit of Interstitial Lung Diseases for telomere shortness as a first grade relative of a patient with IPF. His brother suffered from IPF, and both patients were diagnosed with telomere shortness (<1%) after genetic processing of a mouth swab specimen. He had no respiratory symptoms. Chest radiography showed bilateral peripheral reticulo-nodular pattern. Pulmonary function tests showed a mild restrictive pattern with forced vital capacity (FVC) of 3.83L (79%), total lung capacity (TLC) of 6.43L (85%) and carbon monoxide transfer coefficient (KCO) 4.82 (125%). No oxygen desaturation was seen in the 6-minute walking test. Laboratory data including hematological tests, coagulation, and renal and liver functions were normal. Serum precipitins for different common antigens (avian and fungi) were negative, as were immunological investigations, including rheumatoid factor, antinuclear antibodies and antineutrophil cytoplasmic antibodies. A high-resolution CT chest scan demonstrated profuse calcification within the reticular (septal) lines (Fig. 1A). A surgical lung biopsy of the upper and lower left lobules was performed. Histology showed numerous foci of branching bone tissue, some of them with associated marrow elements, and marked fibrosis of neighboring interstitium with abundant fibroblastic foci, consistent with DPO and probable UIP pattern (Fig. 1B).
Discussion of this clinical case was made after a search of the terms dendriform pulmonary ossification and pulmonary fibrosis in PubMed as of January 6th 2016, and includes citations from 2003 to 2013.
The incidence of DPO in patients with respiratory diseases has been estimated, from autopsy reviews, at between 0.6% and 1.63%, mainly in late stages of chronic obstructive pulmonary disease and some ILD.1 Familiar clustering has also been described in one case of spontaneous pneumothorax in a 29-year-old patient and his otherwise healthy father.2 The incidence of DPO in fibrotic ILD seems to be higher, but the clinical-pathogenic significance remains unknown. Kim et al. retrospectively reviewed 75 cases of UIP, with an incidence of 6.7% of DPO seen in both CT scans and open-lung biopsy specimens. However, they found no cases of DPO in 44 patients with non-specific interstitial pneumonia (NSIP), suggesting that DPO could be useful for the differentiation between UIP and NSIP.3 Nevertheless, the relevance of this finding for the differential diagnosis remains controversial.
Our case demonstrates that DPO can also be found in a very early stage of fibrotic ILD. Since DPO contains bone marrow mesenchymal stem cells (MSCs) and could be a source of different pro-fibrotic mediators, it would be relevant for lung fibrosis development and/or progression. Although the mechanism of bone formation during normal development is clearly defined, the formation of true bone in unusual sites and especially in the lungs is not well understood. It is thought that these two mechanisms may not differ greatly since heterotopic bone is morphologically the same as that found in normal skeleton. The association of FPF or IPF with heterotopic ossification (HO) supports the theory of a genetic linkage in this case. This linkage has been described on the basis of homologous sequences on the transforming growth factor-β (TGF-β) and bone morphogenic protein (BMP) genes. Another explanation could be the Wnt/β-catenin pathway, which controls osteoblast function and bone formation and regulates differentiation of MSCs into myofibroblasts, as well as osteopontin production, which is involved in the induction of fibroblast migration and proliferation, and also in the differentiation of MSCs into osteoblasts.4 Additionally, the recently described Glast-expressing progenitor MSCs, which have been identified as major contributors to HO, could also play a role in the pathogenesis of pulmonary fibrosis.5
The requirement of lung biopsy in FPF patients, mainly in subclinical cases, is a current focus of debate among ILD clinicians. Our case is an example of lung biopsy allowing the final diagnosis of the patient to be defined from early stages of the disease. The procedure may reveal interesting histological findings providing relevant information for better understanding of pathogenic lung fibrotic process.
In conclusion, this case suggests that the presence of HO could be involved in the progression of the lung fibrotic process in lung fibrosis, rather than being only a consequence. The heart of the matter lies, therefore, in understanding the heterogeneous fibrotic pathogenesis in order to identify the appropriate treatment targets for the different IPF or FPF cases.
Please cite this article as: Diez-Ferrer M, Luburich P, Llatjós R, Xaubet A, Dorca J, Molina-Molina M. Osificación pulmonar dendriforme en un caso subclínico de fibrosis pulmonar familiar. Arch Bronconeumol. 2016;52:e9–e10.