With regard to the “Consensus Document on the Diagnosis, Treatment and Prevention of Tuberculosis”, presented by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), published simultaneously with exactly the same contents in the official journals of both societies, Archivos de Bronconeumología and Enfermedades Infecciosas y Microbiología Clínica,1,2 we, as authors of the consensus document, would like to make the following remarks:
- 1.
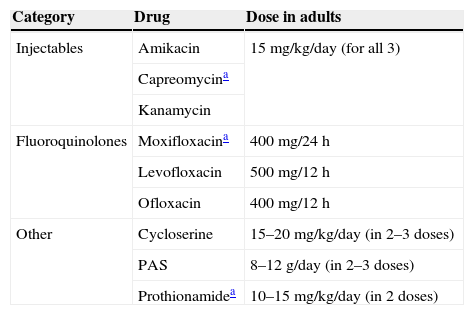
A mistake was detected in the list of recommended doses of para-aminosalicylic acid (PAS) and prothionamide. In Table 11 of the consensus, the doses of both drugs have been interchanged: the dose of prothionamide has been given for the PAS dose, while the dose of PAS has been given for the prothionamide dose.
- 2.
The PAS dose is 8–12g/day, split into 2–3 doses.
- 3.
The dose of prothionamide is 10–15mg/kg/day (in 2 doses).
Changes are given in the attached Table 1.
Second-line drugs in the treatment of tuberculosis.
| Category | Drug | Dose in adults |
|---|---|---|
| Injectables | Amikacin | 15mg/kg/day (for all 3) |
| Capreomycina | ||
| Kanamycin | ||
| Fluoroquinolones | Moxifloxacina | 400mg/24h |
| Levofloxacin | 500mg/12h | |
| Ofloxacin | 400mg/12h | |
| Other | Cycloserine | 15–20mg/kg/day (in 2–3 doses) |
| PAS | 8–12g/day (in 2–3 doses) | |
| Prothionamidea | 10–15mg/kg/day (in 2 doses) |
This table corresponds to Table 11 in the “Consensus Document on the Diagnosis, Treatment and Prevention of Tuberculosis”.1,2
Moreover, regarding the doses for medication used in the treatment of tuberculosis, we would like to recommend the handbooks published by the TB Alliance and the World Health Organization. Links are given below in references 3 and 4.
Thank you for your help in publishing this letter.
Consensus Coordinators representing SEPAR and SEIMC, respectively, on behalf of all authors.
Please cite this article as: García-García J-M, González-Martín J. Dosis de protionamida y PAS. Corrección al «Documento de Consenso sobre diagnóstico, tratamiento y prevención de la tuberculosis». Arch Bronconeumol. 2015;51:533.