The prevalence of COPD among patients treated in the healthcare system in Latin America is unknown. The PUMA study (Prevalencia y práctica habitUal –diagnóstico y tratamiento– en población de riesgo de EPOC en Médicos generalistas de 4 países de América Latina) screened at-risk patients attending primary care centers to evaluate the prevalence, diagnosis, and treatment of COPD in this setting. The aim of this report is to describe the study methodology.
MethodsMulticenter, observational, cross-sectional study conducted in Argentina, Colombia, Uruguay, and Venezuela. Subjects were ≥40 years, smokers, former smokers, and/or exposed to fossil fuels attending primary care centers. Eligible patients underwent pre- and post-bronchodilator spirometry and completed standardized questionnaires on demographics, smoking, exposure to environmental/domestic pollution, symptoms/history, and management of respiratory diseases, comorbidities, and use of healthcare resources.
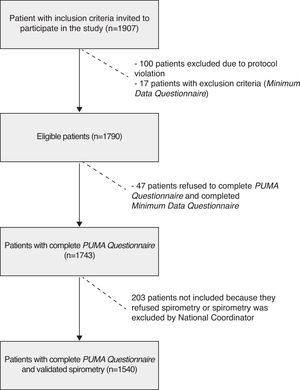
ResultsA total of 57 centers in 4 countries participated; 1907 patients were included, 1743 completed the PUMA questionnaire and 1540 patients underwent validated spirometry.
ConclusionsWe describe the methodology used in the PUMA study, the first systematic multicenter study in four Latin American countries aimed at detecting COPD cases confirmed by spirometry in primary care. Approximately 90% of patients who completed the PUMA questionnaire underwent valid spirometry tests. This gives room for reflection on the feasibility of opportunistic screening at the primary care level to detect patients in the early stages of COPD or with undiagnosed COPD, and improve the diagnosis and management of this disease.
Se desconoce la prevalencia de EPOC entre pacientes que acuden al sistema de salud en Latinoamérica. El estudio Prevalencia y práctica habitUal –diagnóstico y tratamiento– en población de riesgo de EPOC en Médicos generalistas de 4 países de América Latina (PUMA) evalúa prevalencia, diagnóstico y tratamiento de EPOC en pacientes en riesgo que acuden a atención primaria constituyéndose en detección de casos oportunista. El objetivo de esta publicación es describir la metodología del estudio.
MétodosEstudio multicéntrico, observacional, transversal, realizado en Argentina, Colombia, Uruguay y Venezuela. Participaron pacientes ≥40 años, fumadores o ex fumadores y/o expuestos a combustión de biomasa que acudieron a consultas de atención primaria. Los pacientes elegibles realizaron espirometrías pre y posbroncodilatador y respondieron cuestionarios estandarizados sobre datos demográficos, hábito tabáquico, exposición a polución ambiental/doméstica, síntomas/antecedentes y manejo de enfermedades respiratorias, comorbilidades y uso de recursos sanitarios.
ResultadosParticiparon 57 centros en 4 países. Se reclutaron 1.907 pacientes, 1.743 completaron el Cuestionario PUMA y 1.540 pacientes realizaron espirometrías validadas.
ConclusionesDescribimos la metodología empleada en el estudio PUMA, primer estudio sistemático multicéntrico realizado en 4 países de Latinoamérica para detección de casos de EPOC confirmados por espirometría en atención primaria. Aproximadamente el 90% de los pacientes que realizaron el Cuestionario PUMA realizaron espirometrías válidas. Esto permite reflexionar sobre la factibilidad de realizar detección de casos oportunista en el primer nivel asistencial para encontrar pacientes en estadios tempranos o no diagnosticados y mejorar el diagnóstico y manejo de EPOC en atención primaria.
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable condition characterized by progressive, partially reversible airway obstruction that can be associated with dyspnea, cough, and sputum production.1–3 The worldwide impact of COPD is increasing, and it is becoming one of the major causes of mortality, morbidity, and loss of quality of life, contributing to rising social and medical costs.4–6
Early diagnosis and appropriate treatment are essential to prevent COPD and minimize the progress and consequences of this disease.2 Since COPD can be asymptomatic in the early stages and diagnosis requires spirometric evaluation for confirming airway obstruction, underdiagnosis, and misdiagnosis are very common, particularly at disease onset.1–3,6
Numerous COPD population-based studies (PLATINO, BOLD, EPISCAN) have been conducted in different countries, reporting a prevalence of around 10%–15% in the study population.7–9 In the PLATINO study (Chronic Obstructive Pulmonary Disease in Five Latin American Cities), the prevalence among the populations of 5 Latin American cities was, on average, 14.3%.7 In this study, 89% of individuals found to have COPD according to the spirometry results did not have a previous diagnosis, while 64% of subjects who claimed to have COPD showed no airway limitation on spirometry, and only 20% had ever performed spirometry testing at any time in their lives. This suggests that the diagnosis of COPD is severely limited by the underuse of spirometry.10
Less information is available on the prevalence of COPD in the population attending primary care centers. The limited data available in the international literature suggest a significantly higher figure of around 20% in this population.11,12 The only Latin American study conducted in this context reported a prevalence of 20.6% in 27 Mexican cities.13 Early-stage or undiagnosed patients are more likely to be found at the primary care level, therefore the prevalence of the disease at this stage must be determined in order to plan and implement appropriate early detection and management strategies.11,12 Data from prevalence studies are complemented by data from the primary care population. Both are necessary for defining a comprehensive healthcare approach.
Given the prevailing situation, the PUMA study was proposed [Prevalence Study and Regular Practice (Diagnosis and Treatment) Among General Practitioners in Populations at Risk of COPD in Latin America–clinicaltrials.gov identifier: NCT01493544]. This study was designed to estimate the prevalence, determining factors for underdiagnosis and misdiagnosis and treatment of COPD among high-risk patients attending primary care centers for whatever reason in Argentina, Colombia, Uruguay, and Venezuela. The methodology is described below.
MethodsDesign and Study PopulationThe PUMA study is a multicenter, multinational, cross-sectional, non-interventionist study. A steering committee composed of experts in epidemiology and respiratory medicine in Latin America was appointed to design the protocol and standardize the methodology. An expert pulmonologist was also appointed as national coordinator for each country.
Study sites in each country were selected to reflect the reality of national primary care practice in terms of geographical distribution (urban or rural) and healthcare sector (public or private). Participating sites included primary care centers (family doctors, general practitioners, etc.) with no direct connection with respiratory medicine specialists. The study was approved by the ethics committees for each site and all participants gave their informed consent.
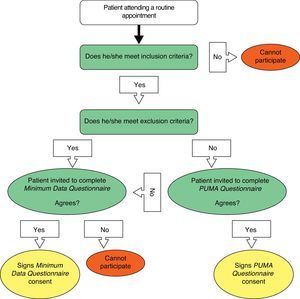
The study population consisted of patients with a high risk of COPD attending participating primary care centers in Argentina, Colombia, Uruguay, and Venezuela. They were enrolled during routine spontaneous or scheduled visits and their medical appointment was unrelated to the study. To be considered high risk, patients had to meet the following inclusion criteria: ≥40 years, current or former smokers (≥10 pack-years, ≥50 pipes/year, or ≥50 cigars/year), and/or exposure to biomass smoke, such as wood or coal, for cooking or heating (exposure ≥100h/year).
Exclusion criteria were pregnancy, contraindication for spirometry (chest, abdominal or brain surgery; acute coronary syndrome; retinal detachment; hospitalization for any heart complaint during the previous 3 months), heart rate ≥120bpm, physical or mental disability rendering the patient unable to complete the study procedures, currently undergoing treatment for tuberculosis or concurrent participation in a clinical trial.
Each center was assigned a number of patients for enrollment. The recruitment period in each center depended on the number of patients with inclusion criteria presenting in the center and completing the study.
Patients were consecutively selected for participation in the study according to the order in which they were seen. Those who did not meet the inclusion criteria continued with their scheduled appointment.
Personnel QualificationsThe national coordinators were responsible for the training and certification of staff involved in completing questionnaires and performing spirometries. This was carried out in each country by way of a training session and use of a standardized evaluation designed specifically for this study, based on the recommendations of the American Thoracic Society (ATS).14 Investigator meetings were held to ensure compliance with the procedures and quality standards. The national coordinators standardized the spirometry procedure and reported and monitored the quality of the maneuvers performed. Personnel in the field sent the spirometries in digital format to the national coordinator within 24h after performance. Approval of the technical quality was required before recruitment in a center could be completed. Only spirometries approved by the coordinator were considered validated. If the spirometry was not validated, it was either repeated as soon as possible or the spirometry for that patient was excluded.
Study ProceduresInvestigators were responsible for selecting the study population according to the inclusion and exclusion criteria and for obtaining informed consent. Data were obtained from the physicians and from the patients on standardized questionnaires (Table 1).
Questionnaires and Visit Activities.
| Study visit | Visit 1 | Visit 2d |
| Patient informed consent | X | |
| Inclusion and exclusion criteria | X | |
| Investigator Questionnairea | X | |
| Minimum Data Questionnaireb | X | |
| PUMA Questionnaire | X | |
| Patient history questionnairec | X | |
| Spirometry | X | X |
| Oximetry | X | X |
The age and sex, years of experience in general medicine, professional training, smoking history, average number of patients seen per day, teaching activities, location of the study center (urban/rural), medical practice characteristics (public/private), factors limiting use of COPD guidelines and available diagnostic and therapeutic resources of each physician were recorded in the Investigator's Questionnaire.
Physicians recorded data from clinical records, including spirometries, diagnoses, previous treatments, and concomitant diseases in the Background Questionnaire.
The physician checked if the patient could perform spirometry and oximetry during their visit. Patients were then referred to personnel qualified to perform the test and complete the corresponding questionnaire. To complete the PUMA Questionnaire (modified version of the PLATINO study questionnaire), the patient was directly interviewed by trained personnel. Patients who not only met the inclusion criteria but also met any of the exclusion criteria were invited to participate in the Minimum Data Questionnaire (basic demographic data and smoking habit).
The primary outcome variable was diagnosis of COPD based on spirometry. Subjects with COPD were defined as those with an FEV1/FVC (forced expiratory volume in 1s divided by forced vital capacity) ratio of less than 0.7. COPD prevalence was also estimated on the basis of other spirometric criteria such as the GOLD classification (http://www.goldcopd.org) and lower limit of normal (LLN) criteria. Independent variables were obtained from the questionnaires and included demographic data, smoking habit, environmental/domestic pollution, symptoms/history, and treatment of respiratory diseases, concomitant diseases and use of healthcare resources.
If the patient presented any transient criterion that would prevent performance of the spirometry, the questionnaire was completed and a second visit was scheduled. Criteria for postponing spirometry included the use of short-acting bronchodilators in the previous 3h, long-acting beta-adrenergic bronchodilators in the previous 12h, long-acting anticholinergics in the previous 24h, smoking cigarettes, pipes or other tobacco products in the previous 2h, physical exercise, including working out, walking or jogging in the previous hour, and concurrent acute respiratory infection at the time of the visit.
Height and weight data were measured using calibrated equipment. Spirometries were obtained in all centers using an ultrasound spirometer (Easy One by NDD TECHNOPARK, Medical Technologies, Inc. Two Dundee Park, Andover, MA, U.S.A.) that met ATS quality control requirements.14
To determine spirometric acceptability and reproducibility, ATS criteria (150–200ml in a minimum of 8 and maximum of 15 maneuvers) were used to obtain reproducible measurements. Spirometries were carried out with the patients in a sitting position using nasal clips. Each participant completed 2 spirometric tests– baseline spirometry examination and a second test 15min after the administration of 400μg of aerosol salbutamol administered with a spacer.
Oximetry was carried out using a digital pulse oximeter after the patient had been resting for 5min and before receiving the bronchodilator for the spirometry (Riestet-ri-fox N pulse Oximeter [Rudolf Riester GmbH – Bruckstrasse 31, D-72417 Jungingen, Germany] or NONIN Onyx 9500 Oximeter [Nonin Medical, Inc., 13700 1st Avenue, North Plymouth, MN, USA]). The evaluation was performed with the patient in a sitting position breathing room air with the oximeter attached to the right index finger. The average of 6 measurements obtained at 10-s intervals was recorded. In addition, the heart rate provided by the device was recorded. The flow chart for patient selection is shown in Fig. 1, and the procedures performed during the visit are described in Table 2.
Patient Numbers by Country.
| Argentina n (%) | Colombia n (%) | Uruguay n (%) | Venezuela n (%) | Total | |
| Time between enrollment of first patient and completion of last patient | 5 months | 4 months | 2 months | 8 months | 8 months (maximum) |
| Final sample size | 506 (26.5) | 476 (25.0) | 112 (5.9) | 813 (42.6) | 1907 (100.0) |
The original size of the sample calculated for studying the prevalence of COPD confirmed by spirometry in the study population of each country (estimated at 20%) with a 3% margin of error was a minimum of 715 subjects per country. The planned sample size was reached only in Venezuela, where 721 patients had completed the PUMA questionnaire by the end of enrollment. Thus, in Argentina and Colombia, with 454 and 465 subjects enrolled, respectively, the margin of error for the estimated prevalence is 3.7%. To increase the sample size for the pooled analysis, 103 patients were included from Uruguay.
In addition to country-specific analyses, some risk factor tests will be performed on the overall study population. In such cases, statistical power is at least 90%, with probability indexes of (a) 1.4 or greater for exposure factors affecting 20% of the disease-free population and (b) 1.6 or greater for exposure factors affecting 10% of the disease-free population. For secondary endpoints restricted to participants with COPD (planned n=572), margins of error for proportions of 10%, 20%, and 50% are 2.5%, 3.5%, and 4.2% respectively.
Descriptive statistics include calculations of means, medians, standard deviations and ranges for continuous variables and proportions and their respective 95% confidence intervals for categorical variables. The prevalence and treatment of COPD will be studied for each country with the exception of Uruguay, due to the small sample size. Most of the remaining analyses will be performed on the pooled sample, except when heterogeneity testing suggests the need for country-specific analyses. In bivariate analyses, proportions will be compared using the Chi-squared test for heterogeneity and linear trend. Differences in means will be investigated with the T-test or one-way ANOVA. For multivariate analyses, logistic and linear regressions will be used for categorical and continuous results, respectively. Statistical analysis will be performed using Stata, version 12 (www.stata.com).
ResultsThe protocol was finalized in August 2011. During the 3rd quarter of 2011 and the 1st quarter of 2012, each participating country obtained approval to conduct the study from their corresponding ethics committees and/or national authorities, depending on local regulations for this type of study. The time required for this process varied in each country, and these differences affected the recruitment period.
The first patient was enrolled in Argentina on February 6, 2012 and the last was completed in Colombia on October 31, 2012 (Table 2). The database closed on November 30, 2012.
The number of centers in each participating country varied, reflecting local primary care practices. A total of 57 centers took part.
In total, 1907 patients were enrolled, of which 1790 were eligible, meeting all of the inclusion criteria and none of the exclusion criteria. One hundred (100) patients were excluded due to violation of inclusion criteria, and 17 met at least one of the exclusion criteria. This latter group completed the Minimum Data Questionnaire.
Of the 1790 eligible patients, 47 refused to complete the PUMA Questionnaire, but did complete the Minimum Data Questionnaire, therefore, a total of 1743 patients completed the PUMA Questionnaire. Of these, 1540 had acceptable spirometries according to the criteria of the national coordinators. Thus, 88.4% of the patients who completed the PUMA Questionnaire had valid spirometries; the prevalence of COPD was assessed in this population (Table 3 and Fig. 2).
Response Rates by Country.
| Argentina n (%) | Colombia n (%) | Uruguay n (%) | Venezuela n (%) | Total n (%) | |
| Eligible patients | 491 (26.3) | 467 (26.1) | 104 (5.8) | 726 (40.6) | 1790 (100.0) |
| All inclusion criteria met but refuses to complete PUMA Questionnaire | 37 (7.5) | 2 (0.4) | 1 (1.0) | 5 (0.7) | 47 (2.5) |
| Minimum Data Questionnaire completed | 42 (8.5) | 11 (2.3) | 2 (1.9) | 9 (1.2) | 64 (3.5) |
| Overall PUMA Questionnaire response rate | 454 (91.5) | 465 (97.7) | 103 (98.1) | 721 (98.8) | 1743 (96.5) |
| Validated spirometries | 446 (98.2) | 326 (70.1) | 103 (100.0) | 665 (92.2) | 1540 (88.4) |
The PUMA study is an observational, multicenter, multinational, cross-sectional study in a patient population at high risk for COPD attending routine or spontaneous visits in a primary care center. Since the study was specifically designed to evaluate the prevalence of spirometry-confirmed COPD in this patient population, it can be termed an opportunistic case screening study.
Different values from those reported in general population studies have been revealed in case-finding studies in primary care, underlining the need for obtaining specific results in this patient group for proper healthcare planning. Laniado-Laborin et al. used case-finding methods among primary care physicians in 27 cities in Mexico.13 They found a 20.6% prevalence of COPD, significantly greater than the rates reported in the PLATINO study for Mexico City (7.8%), in the EPI-SCAN study for Spain (10.2%) and in a review of epidemiological studies (4%–10%), all of which were population-based.7,9,15 In a 3-center primary care study, Hill et al. also found a 20.7% prevalence, of which only 32% were previously aware of their diagnosis.11
One strength of this study is the use of a strict protocol in a predefined population applying a similar study methodology in all centers based on validated questionnaires and standardized spirometry with similar equipment that, moreover, allowed the results to be compared using different spirometric criteria (GOLD, LLN).7,8
The Lanaido-Laborin study did not evaluate a randomized patient sample but rather patients pre-selected by general practitioners, and is therefore prone to bias.13 To avoid such issues in the PUMA study, centers were selected on the basis of available lists of primary care physicians and the study patients were those who visited the center spontaneously. Despite the efforts made to ensure a representative sample, we are aware that there may be some problems regarding the precision of external validity. Nevertheless, the procedure used was the most reasonable in view of the operational possibilities in each country.
The PUMA study will clarify the prevalence of underdiagnosis and misdiagnosis of COPD associated with the underuse of spirometry in the population of 4 Latin American countries. The multicenter, multinational nature of the study is another important aspect, since this will give a comparative overview of several countries studied using the same methodology. The above-mentioned potential external validity issues will need to be addressed in the analysis of results and inter-country variations.
A large amount of additional data has been collected via the PUMA Questionnaire that will be useful for evaluating key issues such as demographic influence and socioeconomic factors, the presence of symptoms, exacerbations and the treatment of respiratory conditions and concomitant diseases impacting on both symptom severity and patient quality of life and the risk of hospitalization and death.15–17 The heterogeneity of all these factors among the participating countries will be analyzed.
This study may also help to elucidate the role of primary care physicians in the appropriate use of healthcare and therapeutic resources, and will assist in the development of strategies for guiding these physicians toward improving the diagnosis and management of COPD.
In the PUMA study, almost 90% of patients who completed the PUMA Questionnaire had valid spirometries performed in primary care. This suggests that it may be possible to use case-finding techniques to achieve earlier diagnosis. There is little doubt that early diagnosis modifies the natural course of a disease that is frequently underdiagnosed and generally remains unidentified until the advanced stages.
Number of words of main part of the manuscript: 2594.
FundingThis observational study was sponsored by AstraZeneca América Latina.
Editorial support was provided by Flow Healthcare Consultancy, funded by AstraZeneca.
Conflict of InterestsEduardo Schiavi has received fees for speaking engagements and/or scientific consultancy from AstraZeneca, Boehringer Ingelheim, and Takeda.
Roberto Stirbulov has received fees for speaking engagements and/or scientific consultancy from Boehringer Ingelheim and Novartis.
Ramón Hernández was an employee of AstraZeneca Latinoamérica during the design and development of the PUMA study.
Sandra Mercurio is an employee of AstraZeneca Argentina.
Valentina Di Boscio is an employee of AstraZeneca Argentina.
Author ContributionES contributed to the concept and design of this study and participated in the writing and critical review of this manuscript. RS contributed to the concept and design of this study. SM contributed to the concept and design of this study and participated in the writing and critical review of this manuscript. VDB contributed to the concept and design of this study and participated in the writing and critical review of this manuscript.
The Steering Committee was formed of members of the Latin American Thorax Association (ALAT) of Argentina, Brazil, Colombia, Mexico, Uruguay, and Venezuela.
National Coordinators: Gustavo Zabert (Argentina), Carlos Aguirre (Colombia), Alejandra Rey (Uruguay), and Dolores Moreno (Venezuela).
Steering Committee: María Montes de Oca (Venezuela), Alejandro Casas (Colombia), Eliseo Guallar (United States), José Jardim (Brazil), María Victorina López Varela (Uruguay), Sandra Mercurio (Argentina), Alejandra Ramírez Venegas (Mexico), Eduardo Schiavi (Argentina), and Roberto Stirbulov (Brazil).
We thank Pedro Hallal for his contribution to the statistical analysis, and all the investigators and patients who participated in the PUMA study.
Please cite this article as: Schiavi E, Stirbulov R, Hernández Vecino R, Mercurio S, Di Boscio V, en nombre del Equipo Puma. Detección de casos de EPOC en atención primaria en 4 países de Latinoamérica: metodología del Estudio PUMA. Arch Bronconeumol. 2014;50:469–474.