Following a proposal by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), sponsor of the Spanish COPD Guidelines (GesEPOC) and the Spanish Guidelines on the Management of Asthma (GEMA), authors of both papers have unified the criteria for the diagnosis of asthma–COPD overlap (ACO).
This consensus defines ACO as the presence in a given patient of three elements: significant smoking exposure, chronic airflow limitation and asthma. Diagnosis is confirmed when a patient (35 years of age or older), smoker or ex-smoker of more than 10 pack-years, presents airflow limitation (post-bronchodilator FEV1/FVC <0.7) that persists after treatment with bronchodilators and inhaled corticosteroids (even after systemic corticosteroids in selected cases), and an objective current diagnosis of asthma (according to GEMA criteria). In cases in which the diagnosis of asthma cannot be demonstrated, marked positive results on a bronchodilator test (FEV1 ≥15% and ≥400ml) or elevated blood eosinophil count (≥300eosinophils/μL) will also be diagnostic of ACO.
The opinion of another 33 experts who had not participated in the consensus was sought using a modified Delphi survey. Up to 80% of respondents gave a very positive opinion of the consensus, and declared that it was better than other previous proposals. The GesEPOC-GEMA consensus on ACO provides a unique perspective of the diagnostic problem, using a simple proposal and a pragmatic diagnostic algorithm that can be applied at any healthcare level.
A instancias de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR), promotora de la Guía española de la EPOC (GesEPOC) y de la Guía Española para el Manejo del Asma (GEMA), autores de ambas guías han unificado criterios diagnósticos del solapamiento asma y EPOC (Asthma-COPD Overlap [ACO]).
Este consenso define al ACO como la coexistencia en un mismo paciente de tres elementos: tabaquismo, limitación crónica al flujo aéreo y asma. La confirmación diagnóstica se establece cuando un paciente (≥35 años) fumador o exfumador (≥10 paquetes-año) presenta obstrucción o limitación crónica al flujo aéreo (FEV1/FVC post-broncodilatador <70%), que persiste tras tratamiento broncodilatador y esteroideo inhalado (incluso oral en casos seleccionados) y diagnóstico objetivo de asma actual (según criterios GEMA). En los casos en los que este último no se pueda establecer, se aceptará una prueba broncodilatadora espirométrica muy positiva (FEV1 ≥15% y ≥400ml) o una elevada eosinofilia en sangre (≥300eosinófilos/μl).
Se solicitó la opinión (mediante encuesta Delphi modificada) a otros 33 expertos que no habían participado en la elaboración del consenso. Un 80% de estos lo valoró positivamente, incluso superior a otras propuestas recientes. El consenso GesEPOC-GEMA sobre ACO proporciona una visión unitaria del problema, con una propuesta conceptual sencilla y un algoritmo diagnóstico pragmático, aplicable en cualquier nivel sanitario de nuestro ámbito.
Asthma and chronic obstructive pulmonary disease (COPD) are different chronic respiratory diseases, but the prevalence of both is high, causing some patients to present both entities concomitantly. The Spanish COPD guidelines (GesEPOC)1 were the first to recognize this phenotype,2 calling it the mixed COPD–asthma phenotype, but since then it has received several names, the most widely recognized nowadays being asthma–COPD overlap, or simply the acronym ACO. From the time it was identified, the notion of overlap has generated considerable debate, and some issues, particularly surrounding concept and diagnosis, remain unclear. Despite the rapprochement of opinions between asthma and COPD experts,3 no uniform criteria are available to define ACO in patients with a previous diagnosis of asthma or COPD. Thus ACO might be defined as an evolving process for which new scientific evidence is still needed to reach definitive conclusions.
Recently, inconsistencies in approaches to ACO proposed in the Spanish reference guidelines for asthma (Spanish Guidelines for the Management of Asthma [GEMA])4 and for COPD (GesEPOC)1 have been pointed out in the different scientific respiratory medicine fora.5 For this reason, on the initiative of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), authors representing both guidelines formed a working group with the aim of reaching consensus on a common definition.
MethodThis Spanish consensus on ACO was executed by the asthma and COPD special interest groups on the initiative of the SEPAR. The coordinators of GEMA and GesEPOC (VP and MM) convened a group of specialists who were involved in drawing up these guidelines, along with a representative from primary care (MRR) who has experience in ACO.
Firstly, the topics of the consensus were defined: concept and definition, epidemiology, diagnostic confirmation, and treatment. Each topic was reviewed by 2 experts, one GEMA representative and one GesEPOC representative. The most important points in each of the sections were discussed in an in-person meeting, and criteria and a diagnostic algorithm for ACO were agreed upon.
Subsequently, the coordinators prepared a questionnaire in which the key points of the proposal were submitted for consensus. The questionnaire, which was reviewed by the whole group, consisted of 20 questions, statements or claims, and was completed online on the SEPAR website (www.separ.es).
A large group of experts in the area of asthma and/or COPD were invited to participate and were given a copy of the draft consensus document. A total of 44 specialists completed the opinion survey, based partly on the Delphi method.6,7 Of these, 29 were respiratory medicine experts, 5 allergologists, 5 primary care physicians, and 5 internal medicine physicians.
The respondents had to score their degree of agreement or disagreement with the wording of the question or statement on a 1–7 Likert scale, in such a way that 1 represented the greatest disagreement with the wording, moving progressively to 7, which represented the greatest agreement. Agreement on the question or statement was consensual when the median score was 6 or 7, and disagreement was consensual when the median was 1 or 2. A median of between 3 and 5 signified a neutral opinion, neither agreement nor disagreement. Participants completed the survey in a first round of questions; in a second round, only questions which did not achieve consensual agreement or disagreement in the first round were addressed. Mean values and standard deviation for the results, percentage of agreement and the percentage of responses with a score of 6 or 7 are shown.
Concept and DefinitionIt is unknown if the overlapping clinical characteristics of COPD and asthma are due to the presence of 2 common diseases in the same patient, or if, in contrast, there is a common underlying pathogenic element. Longitudinal studies recognize childhood asthma as an independent risk factor for developing COPD, particularly when it coincides with smoking.8 However, more than 100 genes that usually code for a lymphocyte T helper (Th2) immunoinflammatory signal and that have been linked with greater reversibility in bronchodilator tests, peripheral eosinophilia, and better response to treatment with inhaled corticosteroids (ICS), have been identified in patients with well-characterized COPD and no history of asthma.9 Despite these findings, insufficient evidence is available to claim a common origin, so the best description of the situation of these patients is overlapping asthma and COPD. Thus, the ACO patient group would include both smokers with asthma who develop persistent airflow obstruction, and COPD patients with characteristics of asthma.10
In general terms, this group of ACO patients has more symptoms, worse quality of life, and greater risk of exacerbations than patients with COPD, although their survival is longer.11–15 Response to treatment with ICS in ACO has also been shown to be halfway between the sensitivity to corticosteroids shown by Th2-high asthmatic phenotypes and the resistance to corticosteroids shown by a large proportion of the COPD phenotypes.14 Differences in clinical outcomes and response to treatment of the asthma and COPD components of ACO require different approaches.
This GesEPOC-GEMA consensus document defines ACO as the presence of persistent airflow limitation in a smoker or former smoker who presents characteristics of asthma. This definition requires the concomitant presence of 3 basic elements: (1) persistent airflow limitation over time, essential to confirm the presence of permanent obstruction that does not change spontaneously or after treatment; (2) accumulated history of smoking (current or past) as a principal risk factor; (3) typical characteristics of asthma, including clinical, biological and functional manifestations.
EpidemiologyThe prevalence of ACO in the general population ranges between 1.6% and 4.5%,10,12–16 in COPD patients between 12.1% and 55.2%,10,12–16 and in patients with asthma, between 13.3% and 61%.17,18 These wide variations reflect the type of population analyzed (analyses of databases or clinical studies), the different criteria used for the identification of ACO, and the definition of asthma and COPD.
In a recently published metaanalysis19 that included 19 studies, the prevalence of ACO among patients with a COPD diagnosis was 27% in population studies and 28% in studies performed in hospital patients. In other recent studies, prevalence ranges from 11% to 25%, depending on the definition.10,20,21 In Spain, the results of the recent CHAIN study, which included 831 patients with COPD from 36 university hospitals, showed a prevalence of ACO (using the specific major and minor modified GesEPOC criteria) of 15%.22 Another 2 observational studies conducted in Spanish populations of 3125 and 331 COPD patients found prevalences of 15.9%23 and 12.1%,21 respectively. These results are similar to those of the COPDGene study, which reported 13%,24 and the MAJORICA study (population cohort of the Balearic Islands), which reported 18.3%.25Table 1 shows the results of the main studies that have evaluated ACO prevalence.
ACO Prevalence According to Diagnostic Criteria.
| Country | Prevalence | Diagnostic Criterion | |
|---|---|---|---|
| Population studies | |||
| De Marco et al.16 (2013) | Italy | 1.6% (20–44 years), 2.1% (45–64 years), 4.5% (65–84 years) | Diagnosis of COPD and asthma |
| Van Boven et al.25 (2016) | Spain | 5.5 per 1000 inhabitants (≥18 years) | Diagnosis of COPD and asthma |
| Rhee et al.38 (2014) | Korea | 54.54% | Diagnosis of COPD and asthma |
| Marsh et al.39 (2008) | USA | 55% | Combination of chronic bronchitis, emphysema and asthma, with and without incomplete airflow reversibility |
| Miravitlles et al.12 (2013) | Spain | 17.4% | Patients with COPD and previous diagnosis of asthma before the age of 40 years |
| Soriano et al.40 (2003) | USA and United Kingdom | 52% | Diagnosis of COPD and asthma |
| Studies of selected patient cohorts | |||
| Cosio et al.22 (2016) | Spain | 15% | Patients diagnosed with COPD with at least 1 of the major criteria (previous history of asthma or bronchodilator response to salbutamol >15% and 400ml) or 2 minor criteria (IGE >100IU, or history of atopy, or bronchodilator response >12% and 200ml on 2 occasions, or eosinophilia in blood >5%) |
| Miravitlles et al.41 (2014) | Spain | 5% | Patients diagnosed with COPD with at least 1 of the major criteria (previous history of asthma, or bronchodilator response to salbutamol >15% and 400ml, or eosinophilia in sputum) or 2 minor criteria (IGE >100IU, or history of atopy, or bronchodilator response >12% and 200ml on 2 occasions, or eosinophilia in blood >5%) |
| Golpe et al.42 (2014) | Spain | 21.3% (COPD with biomass exposure) 5% (COPD with tobacco smoke exposure) | Patients diagnosed with COPD with at least 1 of the major criteria (previous history of asthma, or bronchodilator response to salbutamol >15% and 400ml, or FENO >40ppb) or 2 minor criteria (IGE >100IU, or history of atopy, or bronchodilator response >12% and 200ml on 2 occasions) |
| Kiljander et al.43 (2015) | Finland | 27.4% | Adults with a diagnosis of asthma and smoking (≥10 pack-years) with post-bronchodilator FEV1/FVC ≤0.70 |
| Izquierdo-Alonso et al.21 (2013) | Spain | 12.1% | Adults with a diagnosis of COPD with KCO >80%, no emphysema on imaging tests, and history of asthma before the age of 40 years |
| Miravitlles et al.23 (2015) | Spain | 15.9% | Adults with COPD and a previous diagnosis of asthma |
| Menezes et al.11 (2014) | Latin America | 11.6% | Post-bronchodilator FEV1/FVC <0.7 and asthma (wheezing in the last 12 months plus bronchodilator response of FEV1/FVC 200ml and 12% or medical diagnosis of asthma) |
| Louie et al.44 (2013) | USA | 15.8% in respiratory medicine clinic; 24.3% in asthma clinic | Asthma with partial reversibility of airflow obstruction, with or without emphysema or DLCO <80%; or COPD with emphysema and reversible or partially reversible airflow obstruction, with or without exposure to allergens or reduced DLCO |
| Hardin et al.24 (2011) | USA | 13% | Patients with COPD and previous diagnosis of asthma before the age of 40 years |
| Koblizek et al.45 (2017) | Central and Eastern Europe | 6.9% | Patients with COPD and asthma diagnosed before the age of 40 years, or with a positive bronchodilator response plus atopy |
In short, although the prevalence of ACO varies widely depending on the source and the criteria used to define the syndrome, it seems to lie between 1.6% and 4.5% in the general adult population and between 15% and 25% in the adult population with chronic airflow obstruction.
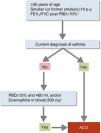
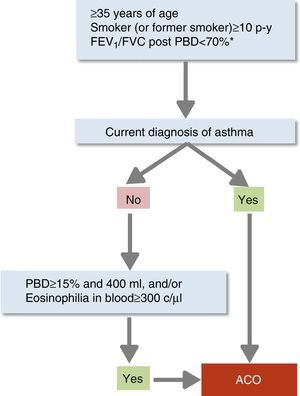
Diagnostic ConfirmationACO diagnosis will be confirmed according to the following stepwise evaluation (Fig. 1):
- 1.
Presence of chronic persistent airflow limitation (FEV1/FVC post-bronchodilator <70%) in a patient ≥35 years, smoker or former smoker, with a smoking history of at least 10 pack-years.1 In recently diagnosed patients, this criterion will be reevaluated after treatment with a long-acting β2-agonist (LABA) and ICS and follow-up of at least 6 months; in some cases, it is also recommendable to administer a short course (15 days) of oral corticosteroids. Reversal of spirometric obstruction after these treatments will rule out the diagnosis of ACO in favor of a diagnosis of asthma.
- 2.
Diagnosis of current asthma.4 Must include: (a) history and/or symptoms causing clinical suspicion: family history of asthma or personal history of asthma in childhood or personal history of atopy (sensitization to certain allergens), with respiratory symptoms (wheezing, cough, chest tightness) of variable course, on occasions in the form of a dyspneic crisis also of variable intensity, or inflammation of the upper airway (rhinosinusitis with or without nasal polyposis); and (b) objective diagnostic confirmation, with reversibility of obstruction of spirometric flows measure by spirometry or positive bronchodilator response (≥12% and ≥200ml), or a diurnal variability of peak expiratory flow (PEF) ≥20%, or exhaled fractional exhaled nitric oxide (FENO) ≥50ppb.
- 3.
If a diagnosis of asthma cannot be established, the ACO diagnosis will be confirmed if the bronchodilator response is very positive (≥15% and ≥400ml), or if eosinophils are observed in blood (≥300eosinophils/μl), or both. These characteristics, while not diagnostic of asthma in themselves, point toward the existence of a Th2-high inflammatory pattern, which allows a smoker with chronic airflow obstruction to be classified as ACO.26
Diagnostic confirmation of asthma–COPD overlap.
*Persistent after treatment with ICS/LABA (6 months). In some cases also after a cycle of oral corticosteroids (15 days).
ACO: asthma–COPD overlap; c: cells; ICS: inhaled corticosteroids; LABA: long-acting β2-agonist; PBD: post-bronchodilator; p-y: pack-years.
Reproduced with permission of the European Respiratory Society ©: Eur Respir J 2017;49:1700068, doi:10.1183/13993003.00068-2017.
In this way, the concept of ACO would encompass patients with a real asthma/COPD overlap, since they share both diagnoses, and to an even greater extent, patients with COPD with asthmatic features defined by an eosinophilic inflammatory component and/or great reversibility of the airflow obstruction.
TreatmentTreatment objectives are also those common to both asthma and COPD individually: to prevent exacerbations, to achieve and maintain acceptable control of symptoms, and to reduce bronchial obstruction.
ICS are the treatment of choice in asthma,4 and patients with COPD and high Th2 expression respond to this treatment.9 Thus, all patients with ACO should receive ICS. A notable increase in the risk of developing pneumonia has been observed with the use of ICS in patients with COPD, particularly at high doses.27 It is unknown if the risk in the ACO patient is similar, so it seems reasonable to assume that the minimum clinically effective dose should be administered. Monotherapy with LABA is contraindicated in asthma, and monotherapy with ICS is contraindicated in COPD. The initial treatment of ACO, then, will be a combination of ICS/LABA. However, very few studies have evaluated the efficacy of these combinations in this setting,28,29 and none have compared them against each other. Consequently, no recommendations can be established regarding the combination of choice.
Tiotropium reduces the risk of COPD30 and asthma exacerbations in patients who do not achieve sufficient control with a combination of ICS/LABA.31 It has also been shown to improve lung function in asthmatics with persistent bronchial obstruction despite treatment with a combination of ICS/LABA, although the impact on symptoms and on quality of life does not appear to be clinically significant.31 Consequently, the addition of tiotropium to a combination of ICS/LABA should be considered if exacerbations and/or significant symptoms persist. No experience has been published on the use in asthma of other long-acting muscarinic agonists (LAMA) that are effective in COPD, such as aclidinium, umeclidium, or glycopyrronium.
Other complementary treatments, such as smoking cessation, respiratory rehabilitation, nasal anti-inflammatories, and oxygen therapy, should also be considered, if indicated.
No solid evidence is currently available to recommend the use of biologics in the treatment of ACO, although their use is recommended in some cases of severe asthma. However, some studies have shown promising results with some of these drugs.32–34
Consensus Opinion SurveyTable 2 lists the results of the analysis of the responses of the participants after the 2 rounds. In the first round, a wide consensus was achieved in 16 of the 20 questions. However, the 4 questions which did not achieve consensus in the first round also failed to achieve consensus in the second.
Questions and Results of the Opinion Survey on the GesEPOC-GEMA ACO Consensus. Questions Which Did Not Achieve Consensual Agreement or Disagreement Among the 44 Respondents Are Indicated in Gray.
| Median | Mean | SD | % Agreement | |
|---|---|---|---|---|
| 1. How far do you agree with the term ACO, instead of ACOS? | 6 | 5.55 | 1.52 | 65.9 |
| 2. The concept of ACO includes patients with different characteristics | 6 | 6.16 | 0.99 | 88.6 |
| 3. A diagnosis of COPD is necessary for a diagnosis of ACO | 7 | 6.43 | 1.28 | 88.6 |
| 4. A diagnosis of asthma is necessary for a diagnosis of ACO | 6 | 5.20 | 1.86 | 56.8 |
| 5. Patients with a diagnosis of COPD and a diagnosis of asthma should be considered ACO | 7 | 6.39 | 1.14 | 88.6 |
| 6. Patients with a diagnosis of COPD and >300 eosinophils in blood should be considered ACO | 3 | 3.59 | 1.58 | 13.6 |
| 7. Eosinophilia in blood must be demonstrated on more than 1 occasion for it to have diagnostic value | 6 | 5.75 | 1.80 | 75.0 |
| 8. Patients with a diagnosis of COPD and a very positive bronchodilator response (>400ml and >15%) should be considered ACO | 6 | 5.09 | 1.68 | 61.3 |
| 9. A very positive bronchodilator response must be demonstrated on more than 1 occasion for it to have diagnostic value | 6 | 5.07 | 1.59 | 52.3 |
| 10. One way of establishing a diagnosis of ACO is the presence of COPD and >300 eosinophils in blood, in addition to a very positive bronchodilator test (>400ml and >15%) | 6 | 5.84 | 1.26 | 70.5 |
| 11. The diagnosis of ACO proposed by GesEPOC in 2012 (major and minor criteria; Arch Bronconeumol 2012;48:331–337) was more appropriate than the GesEPOC-GEMA ACO Consensus | 2 | 2.55 | 1.45 | 52.3 |
| 12. The diagnosis of ACO proposed by GEMA4.0 in 2015 (sequential algorithm of complementary examinations; Arch Bronconeumol 2015;51[S1]:1–68) was more appropriate than the GesEPOC-GEMA ACO Consensus | 3 | 2.90 | 1.59 | 45.5 |
| 13. The diagnostic criteria for ACO in patients initially classified as COPD or asthma may differ | 5 | 4.36 | 1.62 | 13.6 |
| 14. How far do you agree with the strategy of administering a short course of oral corticosteroids to rule out asthma in selected cases? | 6 | 5.18 | 1.93 | 61.3 |
| 15. How far do you agree with the criteria proposed in the GesEPOC-GEMA ACO Consensus for confirmation of the diagnosis of ACO? | 6 | 5.80 | 1.05 | 72.7 |
| 16. ACO patients must receive at least 1 bronchodilator and an inhaled corticosteroid | 7 | 6.75 | 0.53 | 95.4 |
| 17. ACO patients must receive at least 1 bronchodilator, with the addition of an inhaled corticosteroid only in the case of exacerbations | 1 | 1.98 | 1.50 | 79.6 |
| 18. With the current level of evidence, patients with severe ACO are candidates for receiving treatment with biological drugs | 3 | 3.27 | 1.73 | 40.9 |
| 19. Evaluate your level of overall agreement with the GesEPOC-GEMA ACO Consensus proposed here | 6 | 5.77 | 1.24 | 79.6 |
| 20. The conceptual and diagnostic proposals of the GesEPOC-GEMA ACO Consensus are better than those of the recent GOLD 2016 | 6 | 5.98 | 1.28 | 72.8 |
The interpretation of the results of the survey shows that the ACO consensus received a widely positive overall evaluation from the respondents (practically 80% for question 19). The evaluation of this consensus was clearly better than that received by other recent guidelines, especially with regard to conceptual and therapeutic aspects. On the other hand, neither agreement nor disagreement (rejection of the statement) was reached for the proposal to consider a COPD patient with ≥300eosinophils/μl in blood as ACO. This indicates that, while it is widely agreed that a very positive bronchodilator test is an asthmatic feature in a COPD patient,35 and consequently can be considered a criterion for ACO,35,36 raised eosinophilia is not usually perceived as a marker of Th2 inflammation. However, the authors of the consensus agreed to include it in the algorithm as it identifies patients who respond well to ICS.37 Prospective clinical studies are currently ongoing that will help define more precisely the role of eosinophilia in the diagnosis of ACO.
In our opinion, this consensus is a step forward, not only because agreement was reached, but also because the general opinion is that it is an improvement on the previous documents of our respective guidelines. In view of the current evidence, we consider that this consensus offers a rational overview of the problem and a simple, pragmatic diagnostic confirmation, applicable in all of the healthcare levels of our setting, fulfilling the objectives that we set out before undertaking this task.
FundingThis consensus has been funded by a grant from the Integrated Asthma and COPD Research Program and Areas of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).
Conflict of InterestsThe authors received no fees for their participation in this consensus. However:
- -
VP states that in the last 3 years he has received fees for participating as a speaker in meetings organized by Chiesi, Esteve, GlaxoSmithKline, Novartis, Orion and Pfizer, and as a consultant for ALK, MundiPharma, Orion and Teva. He has received economic assistance for attending congresses from AstraZeneca, Chiesi and Novartis, and grants for research projects from AstraZeneca, Chiesi and Menarini.
- -
FA states that in the last 3 years he has acted as a consultant and received assistance for attending congresses and fees for participating as a speaker in various meeting from AstraZeneca, Boehringer Ingelheim, Esteve, GlaxoSmithKline, Novartis, MundiPharma, Pfizer, Sandoz, Teva, and has received grants for research projects from Chiesi, GlaxoSmithKline, Menarini and Novartis.
- -
MC states that she has received assistance for attending congresses and fees for participating as a speaker in various meeting from GlaxoSmithKline, Novartis, and Pfizer, and has received grants for research projects from AstraZeneca and Menarini.
- -
CC states that in the last 3 years he has received fees for speaking engagements and/or scientific consultancy from AstraZeneca, Boehringer-Ingelheim, Gebro Pharma, GlaxoSmithKline, Laboratorios Esteve, Menarini, Novartis and Rovi.
- -
BGC states that in the last 3 years he has acted as a consultant, received assistance for attending congresses and received fees for participating as a speaker in various meeting from GlaxoSmithKline, Novartis, Chiesi, Boehringer-Ingelheim, Menarini and Pfizer.
- -
ALV states that in the last 3 years he has acted as a consultant, received assistance for attending congresses and received fees for participating as a speaker in various meeting from TEVA, GlaxoSmithKline, Novartis, MundiPharma, Chiesi, Boehringer-Ingelheim, and Pfizer.
- -
LPLL states that in the last 3 years he has received payment from Novartis, Astra, Boehringer Ingelheim, Teva, Sanofi, Sandoz, Zambón, Boehringer Ingelheim, Chiesi, Pfizer, Almirall, MundiPharma, Esteve and Ferrer for presentations at medical congresses, consultancy, and coordination or participation in clinical research projects. He has also been invited to attend national and international congresses by some of these laboratories.
- -
SQ states that in the last 3 years he has received fees for participating as a speaker in meetings organized by CAstraZeneca, Chiesi, GlaxoSmithKline, Novartis AND Leti, and as a consultant for ALK, MundiPharma and Teva.
- -
MRR states that in the last 3 years he has received fees for participating as a speaker in meetings sponsored by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, MundiPharma, Novartis, Pfizer, Rovi and Teva, and has received grants for research projects from GlaxoSmithKline and AstraZeneca.
- -
JJSC has received fees for scientific consultancy and/or for speaking at conferences from AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Laboratorios Esteve, Menarini, MundiPharma, Novartis and Rovi.
- -
MM has received fees for scientific consultancy and/or for speaking at conferences from AstraZeneca, Boehringer Ingelheim, CSL Behring, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Teva, Cipla, Menarini, Novartis and Gebro Pharma.
We thank Jordi Giner (Department of Respiratory Medicine, Hospital de la Santa Creu i Sant Pau, Barcelona) for his invaluable contribution to the technical aspects of the consensus opinion survey.
Agüero, Ramón (Neumología, H. U. Marqués de Valdecilla, Santander).
Alcázar, Bernardino (Neumología, H. de Alta Resolución EPHP, Loja, Granada).
Almagro, Pere (Medicina Interna, H. Mutua de Terrassa, Terrassa, Barcelona).
Almonacid, Carlos (Neumología, H. U. Ramón y Cajal, Madrid).
Ancochea, Julio (Neumología, H. U. de la Princesa, Madrid).
Boixeda, Ramón (Medicina Interna, Servicio de Medicina Interna Hospital de Mataro¿, Mataró, Barcelona).
Carretero, José Ángel (Neumología, H. Royo Villanova, Zaragoza).
Cisneros, Carolina (Neumología, H. U. de la Princesa, Madrid).
Delgado, Julio (Alergología, H. Virgen Macarena, Sevilla).
Entrenas, Luis Manuel (Neumología, H. U. Reina de Sofía, Córdoba).
Esteban, Cristóbal (Neumología, H. de Galdakao, Usansolo, Vizcaya).
Fernández Villar, José Alberto (H. Álvaro Qunqueiro, Vigo, Pontevedra).
Gómez, María (Medicina Interna, H. G. U. Gregorio Marañón, Madrid).
Ignacio García, José María (Neumología, H. Quirón, Marbella, Málaga).
Izquierdo-Alonso, José Luis (Neumología, H. U. Guadalajara).
López-Campos, José Luis (Neumología, H. U. Virgen del Rocío, Sevilla).
López-García, Francisco (Medicina Interna, H. G. U. de Elche, Elche, Alicante).
Marín, José María (Neumología, H. U. Miguel Servet, Zaragoza).
Martínez-Moragón, Eva (Neumología, H. U. Dr. Peset, Valencia).
Molina, Jesús (Atención Primaria, Francia I, Fuenlabrada, Madrid).
Muñoz, Xavier (Neumología, H. G. U. Vall d’Hebron, Barcelona).
Olaguíbel, José María (Alergología, C. H. de Navarra, Pamplona).
Quintano, José Antonio (Atención Primaria, Centro de Salud Lucena I, Lucena, Córdoba).
Recio, Jesús Pedro (Medicina Interna, H. G. U. Vall d’Hebron, Barcelona).
Riesco, Juan Antonio (Neumología, H. San Pedro de Alcántara, Cáceres).
Sabadell, Carles (Neumología, H. de Figueres, Girona).
Sastre, Joaquín (Alergología, Fundación Jiménez Díaz, Madrid).
Serrano, José (Neumología, H. Comarcal d’Inca, Inca, Mallorca).
Simonet, Pere (Atención Primaria. EAP Viladecans-2, Viladecans, Barcelona).
Torrego, Alfons (Neumología, H. de la Santa Creu i Sant Pau, Barcelona).
Trigueros, Juan Antonio (Atención Primaria, Centro de Salud de Menasalbas, Menasalbas, Toledo).
Urrutia, Isabel (Neumología, H. de Galdakao, Vizcaya).
Valero, Antonio (Alergología, H. Clínic, Barcelona).
Please cite this article as: Plaza V, Álvarez F, Calle M, Casanova C, Cosío BG, López-Viña A, et al. Consenso sobre el solapamiento de asma y EPOC (ACO) entre la Guía española de la EPOC (GesEPOC) y la Guía Española para el Manejo del Asma (GEMA). Arch Bronconeumol. 2017;53:443–449.