Clinical presentation and short-term prognosis of patients with travel-associated acute pulmonary embolism (PE) (i.e., economy class syndrome [ECS]) is not well understood.
MethodsIn this retrospective cohort study of patients with acute PE identified from a single center registry, we assessed the clinical presentation and the association between ECS and the outcomes of all-cause mortality, PE-related mortality, nonfatal venous thromboembolism and nonfatal major bleeding rates through 30days after initiation of PE treatment.
ResultsOf the 2333 patients with acute symptomatic PE, 124 (5.3%; 95% confidence interval, 4.4%–6.3%) had ECS. Patients with ECS were younger and had fewer comorbid diseases (recent bleeding, chronic obstructive pulmonary disease, congestive heart failure), but they presented with more signs of clinical severity (syncope [48% vs 14%; P<.001], tachycardia [37% vs 21%; P<.001], right ventricular dysfunction [31% vs 19%; P<.01] and myocardial injury [57% vs 28%; P<.001]) compared to those without ECS. Regression analyses showed a significantly lower risk of all-cause mortality for patients with ECS compared to patients without ECS (1.6% vs 9.6%; P<.01). We did not detect a difference in PE-related mortality at 30days between those with and those without ECS (0.8% vs 3.1%; P=.18).
ConclusionsPE patients with ECS are younger and have fewer comorbid diseases compared to those without ECS. Though they present with more signs of clinical severity, their short-term prognosis is excellent.
No se conocen suficientemente las características clínicas y el pronóstico de los pacientes con tromboembolia de pulmón (TEP) aguda sintomática asociada a los viajes prolongados (síndrome de clase turista [SCT]).
MétodosSe analizaron retrospectivamente las características basales de los pacientes con TEP aguda y se estratificaron según el factor de riesgo desencadenante. Se determinaron la mortalidad por todas las causas, la mortalidad por la propia TEP, las recurrencias trombóticas no fatales y los sangrados mayores no fatales durante los primeros 30días de seguimiento.
ResultadosDe los 2.333 pacientes incluidos, un total de 124 (5,3%; intervalo de confianza del 95%: 4,4–6,3) fueron diagnosticados de TEP secundaria a SCT. Estos pacientes fueron más jóvenes, presentaron menos frecuentemente comorbilidad y más frecuentemente síncope (48% vs 14%; p<0,001), taquicardia (37% vs 21%; p<0,001), disfunción de ventrículo derecho (VD) (31% vs 19%; p<0,01) y daño miocárdico (57% vs 28%; p<0,001) que los demás pacientes con TEP. La mortalidad por todas las causas a 30días fue significativamente menor para los pacientes con TEP secundaria a SCT (1,6% vs 9,6%; p<0,01). La mortalidad a 30días por TEP no fue diferente entre los dos grupos de pacientes (0,8% vs 3,1%; p=0,18).
ConclusionesLos pacientes con TEP y SCT son más jóvenes y tienen menos comorbilidad que los demás pacientes con TEP. Aunque se presentan más frecuentemente con disfunción de VD y daño miocárdico, el pronóstico a corto plazo es excelente.
Pulmonary embolism (PE) is a disease with a wide spectrum of clinical manifestations, prognoses, and treatments.1 Depending on the patient's hemodynamic status and right ventricular function at the time of diagnosis, the disease is classified as high risk PE (formerly called massive PE), which is characterized by the presence of arterial hypotension or shock, intermediate risk PE (formerly submassive PE) that occurs in normotensive patients with right ventricular dysfunction and myocardial damage, or low risk PE, in which the patient is hemodynamically stable and right ventricular function is normal.2–4
However, the short-term prognosis of patients with acute symptomatic PE depends not only on the severity of the presentation of the PE, but also on the patient's baseline characteristics and the factor that triggered the thrombotic event. For example, patients with PE associated with cancer have a significantly poorer prognosis than patients with PE due to medical immobilization,5 while patients with PE due to major surgery have the best short-term prognosis.6 Several studies have shown that prolonged air travel (economy class syndrome [ECS]) is a risk factor for acute symptomatic PE.7–9 However, the clinical presentation and the short-term prognosis of patients with ECS is still poorly defined.
The aim of this study was to analyze the baseline characteristics of a cohort of patients with acute symptomatic PE, stratified according to the causative risk factor (ECS vs others). We also compared the short-term prognosis of patients with PE due to ECS with that of patients with idiopathic PE, or PE caused by cancer, immobilization, or surgery.
MethodDesignWe conducted an observational, retrospective study to examine the baseline characteristics and short-term prognosis of a cohort of patients with stable and unstable acute symptomatic PE.
Patients and Selection CriteriaAll patients with a diagnosis of symptomatic acute PE from the emergency department of the Hospital Ramon y Cajal (Madrid, Spain) between January 2003 and June 2016 were included consecutively. The diagnosis of PE was confirmed by computed tomography (CT) angiography findings of a partial or complete intraluminal defect surrounded by contrast medium or complete occlusion of a pulmonary artery in 2 consecutive CT slices.10 PE was diagnosed by ventilation/perfusion scintigraphy in patients with a high probability of PE according to PIOPED criteria11 (at least 1 segmental perfusion defect or 2 subsegmental defects with normal ventilation), or with clinical suspicion of PE, an inconclusive scintigraphy and diagnostic ultrasound of the lower limbs showing incomplete compressibility of the venous lumen as a sign of deep vein thrombosis (DVT).12
InterventionsPatients received low molecular weight heparin (LMWH) at weight-adjusted doses every 12h for at least 5 days. Vitamin K antagonists were started along with LMWH between day 1 and day 3 of treatment, and LMWH was suspended when the international normalized ratio (INR) was stable and greater than 2.0. INR levels were monitored in accordance with the local practices of the center.
Recanalization treatment (thrombolytics, fragmentation or embolectomy) was used in hemodynamically unstable patients at the discretion of the treating physician. In general, mechanical fragmentation and embolectomy were reserved for unstable patients with contraindications for thrombolysis. A vena cava filter was inserted in patients with contraindication for anticoagulation (active bleeding or high risk of bleeding).
Definitions According to Causative FactorStudy patients were classified into one or more of the following groups:ECS, in patients who had made a journey of more than 4h duration in the month before the diagnosis of PE, irrespective of the mode of transport.Cancer, active or in treatment, in the year prior to the diagnosis of PE.Surgery in the month prior to the diagnosis of PE.Immobilization, in non-surgical patients bedridden for 4 or more days in the month prior to the diagnosis of PE.Pregnancy, postpartum period, use of oral contraceptives in the month prior to the diagnosis of PE.Idiopathic, in the absence of any of the above-mentioned triggers.
Study EpisodesThe primary endpoint was defined as all-cause mortality in the month prior to diagnosis. Secondary endpoints were death due to the PE itself, objectively confirmed non-fatal thromboembolic relapse, and non-fatal major bleeding in the month following diagnosis.
Diagnostic criteria of non-fatal thrombotic recurrence were the presence of a new intraluminal defect on CT angiogram, or a new ventilation/perfusion defect on lung scintigraphy; new non-compressible venous segment or increase in the diameter of the thrombus by at least 4mm on lower limb ultrasonography.13
Non-fatal major bleeding was defined as bleeding requiring transfusion of at least 2 units of packed red blood cells, bleeding requiring surgery, or brain, retroperitoneal, or joint bleeding.14
Statistical AnalysisContinuous variables were expressed as mean±standard deviation or median (interquartile range), as appropriate, and were compared with the Student t test or the Mann–Whitney U test for asymmetric data. Categorical variables were represented as percentages and compared using the Chi-square test or Fisher's exact test, if necessary.
Accumulated incidence curves were estimated using the Kaplan–Meier method and compared using the log rank test.15,16 A multivariate logistic regression model was constructed to evaluate the possible association between ECS and mortality.17 A maximum model of 14 variables selected on the basis of the published experience and expert opinion was developed. Variables were: (a) age; (b) sex; (c) chronic obstructive pulmonary disease (COPD); (d) congestive heart failure (CHF); (e) heart rate; (f) systolic blood pressure; (g) dyspnea; (h) chest pain; (i) syncope; (j) ECS; (k) cancer; (l) recent surgery; (m) immobilization, and (n) simplified Pulmonary Embolism Severity Index (PESI) scale.18 The analysis was constructed on the basis of the maximum model and by backward elimination of variables. Confounding variables (i.e., the coefficient of the variable evaluated was modified by more than 10% when the confounding variable was eliminated from the model) of the effect of ECS on the primary endpoint were maintained in the model. Odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated.
SPSS software (version 19.0, SPSS Inc., Chicago, Illinois, USA) was used for the statistical analysis. Results with a 2-tailed value of P<.05 were considered statistically significant.
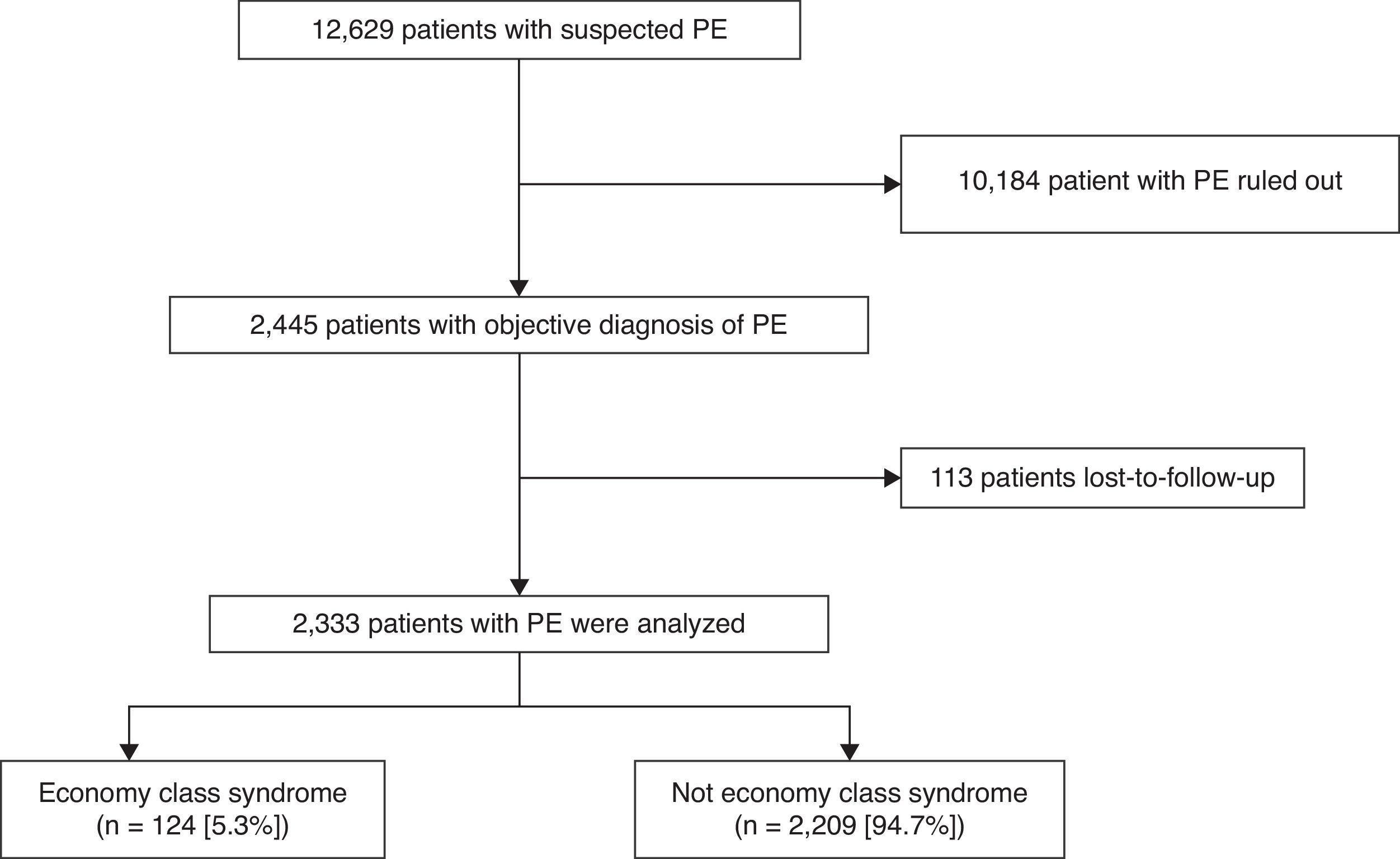
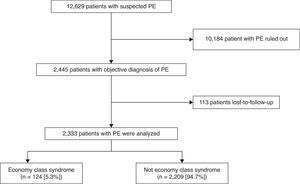
ResultsPatientsDuring the study period, 12629 patients with suspected acute symptomatic PE were evaluated and diagnosis was confirmed in 2445 (19.4%). Of these, 113 were lost to follow-up, so the final sample consisted of 2333 patients (1089 men and 1244 women), 95% of the population evaluated (Fig. 1). No statistically significant differences were found between the baseline variables of the patients who were included and those who were excluded. Approximately 70% of patients (1659/2333) were diagnosed by chest CT angiogram, 744 (32%) by high probability ventilation/perfusion scintigraphy, while 76 (3%) had negative chest studies and DVT confirmed by ultrasound of the lower limbs. Some patients had several simultaneous positive diagnostic tests.
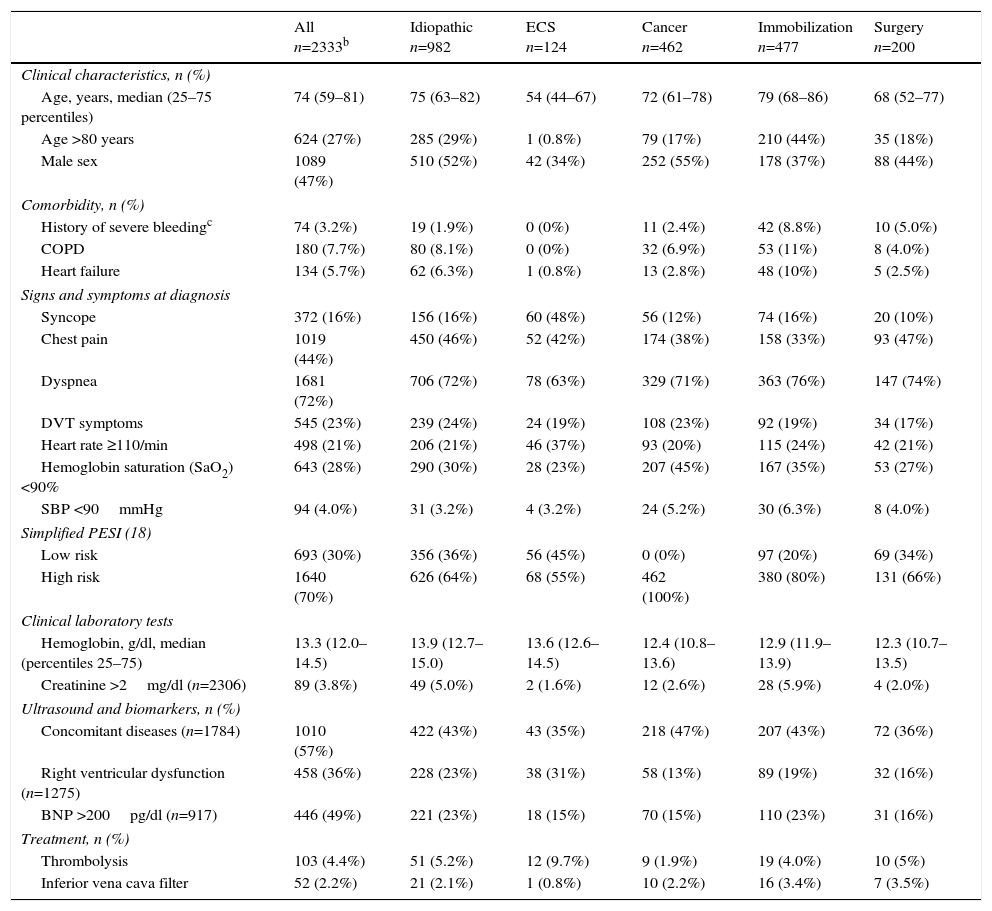
Study patient characteristics are shown in Table 1. The number of patients treated with inferior vena cava filters was low (2.2%; 52 of 2333 patients). Four percent (103 of 2333 patients) were treated with thrombolytics. Fewer patients in the ECS group were men (34% versus 47%; P<.01), and ECS patients were younger (55.8±14.2 vs 69.6±16.5; P<.001) and had less COPD (0% vs 8.1%; P<.001), heart failure (0.8% vs 6.0%; P<.01), cancer (4.8% vs 21%; P<.001), and history of recent hemorrhage (0% vs 3.4%; P=.03) A higher proportion of patients with ECS was classified as low risk according to the simplified PESI scale (45% vs 29%; P<.001). PE was more severe in patients with ECS, who presented more frequently with syncope (48% versus 14%; P<.001), tachycardia (37% vs 21%; P<.001), right ventricular dysfunction (31% vs 19%; P<.01), and myocardial damage (57% vs 28%; P<.001) than patients with idiopathic PE or PE due to other etiologies. More patients with ECS had residual DVT (45% vs 57%; P=.02). With regard to treatment, more patients with ECS received thrombolytic treatment (9.8% vs 4.1%; P<.01).
Baseline Characteristics and Treatment of Study Subjects.a
| All n=2333b | Idiopathic n=982 | ECS n=124 | Cancer n=462 | Immobilization n=477 | Surgery n=200 | |
|---|---|---|---|---|---|---|
| Clinical characteristics, n (%) | ||||||
| Age, years, median (25–75 percentiles) | 74 (59–81) | 75 (63–82) | 54 (44–67) | 72 (61–78) | 79 (68–86) | 68 (52–77) |
| Age >80 years | 624 (27%) | 285 (29%) | 1 (0.8%) | 79 (17%) | 210 (44%) | 35 (18%) |
| Male sex | 1089 (47%) | 510 (52%) | 42 (34%) | 252 (55%) | 178 (37%) | 88 (44%) |
| Comorbidity, n (%) | ||||||
| History of severe bleedingc | 74 (3.2%) | 19 (1.9%) | 0 (0%) | 11 (2.4%) | 42 (8.8%) | 10 (5.0%) |
| COPD | 180 (7.7%) | 80 (8.1%) | 0 (0%) | 32 (6.9%) | 53 (11%) | 8 (4.0%) |
| Heart failure | 134 (5.7%) | 62 (6.3%) | 1 (0.8%) | 13 (2.8%) | 48 (10%) | 5 (2.5%) |
| Signs and symptoms at diagnosis | ||||||
| Syncope | 372 (16%) | 156 (16%) | 60 (48%) | 56 (12%) | 74 (16%) | 20 (10%) |
| Chest pain | 1019 (44%) | 450 (46%) | 52 (42%) | 174 (38%) | 158 (33%) | 93 (47%) |
| Dyspnea | 1681 (72%) | 706 (72%) | 78 (63%) | 329 (71%) | 363 (76%) | 147 (74%) |
| DVT symptoms | 545 (23%) | 239 (24%) | 24 (19%) | 108 (23%) | 92 (19%) | 34 (17%) |
| Heart rate ≥110/min | 498 (21%) | 206 (21%) | 46 (37%) | 93 (20%) | 115 (24%) | 42 (21%) |
| Hemoglobin saturation (SaO2) <90% | 643 (28%) | 290 (30%) | 28 (23%) | 207 (45%) | 167 (35%) | 53 (27%) |
| SBP <90mmHg | 94 (4.0%) | 31 (3.2%) | 4 (3.2%) | 24 (5.2%) | 30 (6.3%) | 8 (4.0%) |
| Simplified PESI (18) | ||||||
| Low risk | 693 (30%) | 356 (36%) | 56 (45%) | 0 (0%) | 97 (20%) | 69 (34%) |
| High risk | 1640 (70%) | 626 (64%) | 68 (55%) | 462 (100%) | 380 (80%) | 131 (66%) |
| Clinical laboratory tests | ||||||
| Hemoglobin, g/dl, median (percentiles 25–75) | 13.3 (12.0–14.5) | 13.9 (12.7–15.0) | 13.6 (12.6–14.5) | 12.4 (10.8–13.6) | 12.9 (11.9–13.9) | 12.3 (10.7–13.5) |
| Creatinine >2mg/dl (n=2306) | 89 (3.8%) | 49 (5.0%) | 2 (1.6%) | 12 (2.6%) | 28 (5.9%) | 4 (2.0%) |
| Ultrasound and biomarkers, n (%) | ||||||
| Concomitant diseases (n=1784) | 1010 (57%) | 422 (43%) | 43 (35%) | 218 (47%) | 207 (43%) | 72 (36%) |
| Right ventricular dysfunction (n=1275) | 458 (36%) | 228 (23%) | 38 (31%) | 58 (13%) | 89 (19%) | 32 (16%) |
| BNP >200pg/dl (n=917) | 446 (49%) | 221 (23%) | 18 (15%) | 70 (15%) | 110 (23%) | 31 (16%) |
| Treatment, n (%) | ||||||
| Thrombolysis | 103 (4.4%) | 51 (5.2%) | 12 (9.7%) | 9 (1.9%) | 19 (4.0%) | 10 (5%) |
| Inferior vena cava filter | 52 (2.2%) | 21 (2.1%) | 1 (0.8%) | 10 (2.2%) | 16 (3.4%) | 7 (3.5%) |
BNP, brain natriuretic peptide; cTnI, cardiac troponin I; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; ECS, economy class syndrome; PESI, Pulmonary Embolism Severity Index; SBP, systolic blood pressure.
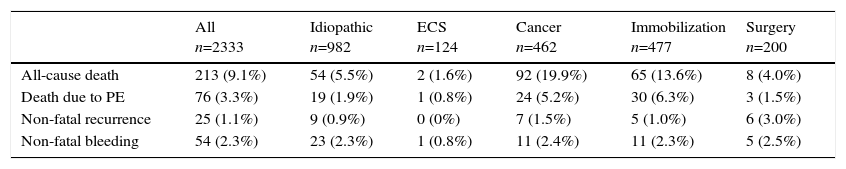
Of the 2333 patients, 213 (9.1%; 95% CI: 8.0–10.4) died during the first 30 days of follow-up (Table 2). Seventy-six patients (36%) died due to their PE, 59 (28%) due to cancer, 25 (12%) due to infection, 10 (4.7%) due to heart failure, 8 (3.8%) due to bleeding, and 35 (16%) due to other causes. Seventy-five patients presented a secondary event: 21 had an objectively confirmed thrombotic recurrence, 50 patients had non-fatal major bleeding, and 4 presented simultaneous bleeding and recurrence.
Mortality and Non-Fatal Adverse Events During the First 30 Days of Follow-Up.a
| All n=2333 | Idiopathic n=982 | ECS n=124 | Cancer n=462 | Immobilization n=477 | Surgery n=200 | |
|---|---|---|---|---|---|---|
| All-cause death | 213 (9.1%) | 54 (5.5%) | 2 (1.6%) | 92 (19.9%) | 65 (13.6%) | 8 (4.0%) |
| Death due to PE | 76 (3.3%) | 19 (1.9%) | 1 (0.8%) | 24 (5.2%) | 30 (6.3%) | 3 (1.5%) |
| Non-fatal recurrence | 25 (1.1%) | 9 (0.9%) | 0 (0%) | 7 (1.5%) | 5 (1.0%) | 6 (3.0%) |
| Non-fatal bleeding | 54 (2.3%) | 23 (2.3%) | 1 (0.8%) | 11 (2.4%) | 11 (2.3%) | 5 (2.5%) |
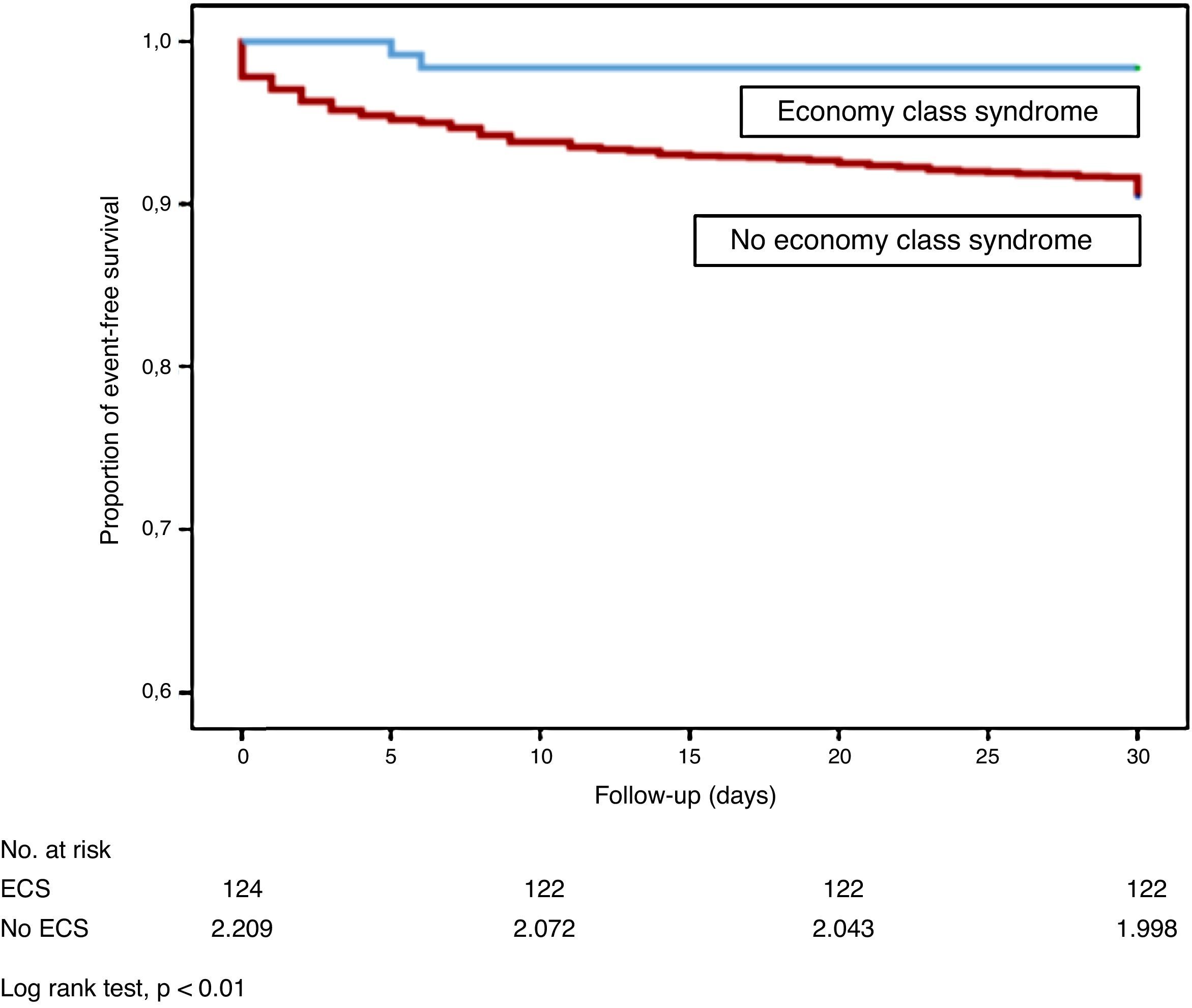
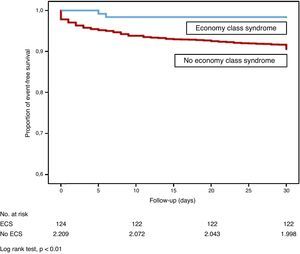
Patients with ECS had a significantly lower risk of all-cause death than patients with idiopathic PE or PE from other causes (log rank test, P<.01; Fig. 2). Mortality due to the PE itself in the first month of follow-up was similar in ECS and non-ECS patients (log rank test, P=.11). None of the ECS patients had thromboembolic recurrence, and only 1 (0.8%) had non-fatal major bleeding in the first 30 days of follow-up.
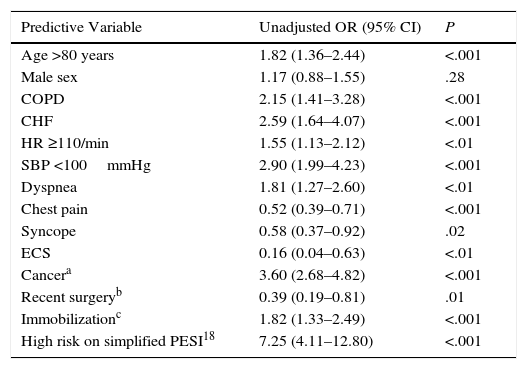
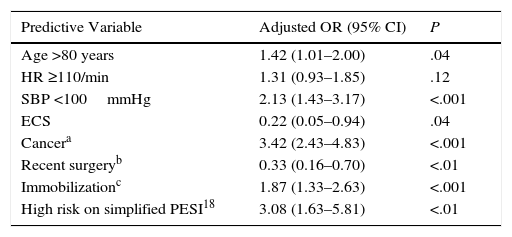
In the univariate analysis, age >80 years (OR: 1.82; 95% CI: 1.36–2.44; P<.001), some comorbidities, including COPD (OR: 2.15; 95% CI: 1.41–3.28; P<.001), heart failure (OR: 2.59; 95% CI: 1.64–4.07; P<.001), cancer (OR: 3.60; 95% CI: 2.68–4.82; P<.001), together with medical immobilization (OR: 1.82; 95% CI: 1.33–2.49; P<.001), clinical presentation [tachycardia (OR: 1.55; 95% CI: 1.13–2.12; P<.01) and hypotension (OR: 2.90; 95% CI: 1.99–4.23; P<.001)], and high risk on the simplified PESI scale (OR: 7.25; 95% CI: 4.11–12.80; P<.001) significantly increased the risk of all-cause death in the 30 days after PE diagnosis (Table 3). In contrast, a history of recent surgery (OR: 0.39; (95% CI: 0.19–0.81; P<.01) and ECS (OR: 0.16; 95% CI: 0.04–0.63; P<.01) were associated with a statistically significant reduction in the risk of death. In the multivariate analysis, ECS was independently associated with all-cause death during the first 30 days of follow-up (OR: 0.22; 95% CI: 0.05–0.94, P=.04) (Table 4). ECS was not independently associated with death due to the PE itself during the first 30 days of follow-up (OR: 0.37; 95% CI: 0.05–2.77; P<.33).
Univariate Analysis for All-Cause Mortality in PE Patients.
| Predictive Variable | Unadjusted OR (95% CI) | P |
|---|---|---|
| Age >80 years | 1.82 (1.36–2.44) | <.001 |
| Male sex | 1.17 (0.88–1.55) | .28 |
| COPD | 2.15 (1.41–3.28) | <.001 |
| CHF | 2.59 (1.64–4.07) | <.001 |
| HR ≥110/min | 1.55 (1.13–2.12) | <.01 |
| SBP <100mmHg | 2.90 (1.99–4.23) | <.001 |
| Dyspnea | 1.81 (1.27–2.60) | <.01 |
| Chest pain | 0.52 (0.39–0.71) | <.001 |
| Syncope | 0.58 (0.37–0.92) | .02 |
| ECS | 0.16 (0.04–0.63) | <.01 |
| Cancera | 3.60 (2.68–4.82) | <.001 |
| Recent surgeryb | 0.39 (0.19–0.81) | .01 |
| Immobilizationc | 1.82 (1.33–2.49) | <.001 |
| High risk on simplified PESI18 | 7.25 (4.11–12.80) | <.001 |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ECS, economy class syndrome; HR, heart rate; OR, odds ratio; PESI, Pulmonary Embolism Severity Index; SBP, systolic blood pressure.
Multivariate Analysis for All-Cause Mortality in PE Patients.
| Predictive Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Age >80 years | 1.42 (1.01–2.00) | .04 |
| HR ≥110/min | 1.31 (0.93–1.85) | .12 |
| SBP <100mmHg | 2.13 (1.43–3.17) | <.001 |
| ECS | 0.22 (0.05–0.94) | .04 |
| Cancera | 3.42 (2.43–4.83) | <.001 |
| Recent surgeryb | 0.33 (0.16–0.70) | <.01 |
| Immobilizationc | 1.87 (1.33–2.63) | <.001 |
| High risk on simplified PESI18 | 3.08 (1.63–5.81) | <.01 |
ECS, economy class syndrome; HR, heart rate; OR, odds ratio; PESI, Pulmonary Embolism Severity Index; SBP, systolic blood pressures.
This study analyzed the clinical presentation and short-term prognosis of ECS in a large series of patients with acute symptomatic PE. Our results indicate that PE in patients with ECS more frequently causes right ventricular dysfunction and myocardial damage than idiopathic PE or PE triggered by other causes. However, the short-term prognosis of these patients is excellent.
We found that, while patients with PE caused by ECS are younger and have less comorbidity (e.g., COPD, heart failure) than patients with idiopathic PE or PE due to other causes, its form of presentation is more severe, and it began more frequently with syncope, tachycardia, and hypotension, all factors for poor prognosis in the acute phase of the PE.19–22 Interestingly, right ventricular dysfunction and myocardial necrosis were significantly more frequent in these patients. These results are similar to those of a previous study that evaluated the form of presentation of patients with ECS.23 The reasons why these patients present this way remain unclear, but some mechanisms may be responsible. Hypoxia in the aircraft cabin increases pulmonary arterial pressure and could worsen right ventricular function.24 Residual DVT was less frequent in patients with ECS than in patients with idiopathic PE or PE triggered by other causes, perhaps because in these patients the entire clot formed in the lower limbs breaks free, causing PE with a larger clot burden.
Despite their more severe PE, the short-term prognosis of these patients is excellent, and significantly better than that of patients with PE due to cancer or immobilization. This can be explained by the fact that while early death (in the initial days) among patients with PE is a result of right ventricular failure, late mortality is the result of patient comorbidity.25
What is the clinical significance of this study? Clinical practice guidelines advise against the use of fibrinolytic treatment in patients with intermediate risk PE. The PEITHO study analyzed the efficacy and safety of treatment with tenecteplase in normotensive patients with right ventricular dysfunction and myocardial damage. Although fibrinolytics significantly reduced the hemodynamic collapse of these patients, mortality was not affected, and severe bleeds were significantly more frequent in those who received fibrinolysis.26 Our results support this recommendation in the subgroup of patients with PE due to ECS. Although this presentation is more serious (e.g., intermediate-high risk PE), prognosis is excellent when conventional anticoagulation is administered.
Our study has some limitations. Firstly, as this is an analysis of a historical cohort composed of patients from a single hospital, it is subject to the biases inherent to this type of study and results are not generalizable. However, it represents an unselected population of PE patients seen in routine clinical practice in our setting, so it may be considered an ideal series for evaluating the form of presentation and prognosis of patients with ECS. Secondly, we could not measure the clot burden of our patients, so we cannot establish an association between the degree of pulmonary artery obstruction and the presence or absence of right ventricular dysfunction.
In conclusion, our results show that patients with PE caused by ECS have less comorbidity than patients with idiopathic PE or PE due to other causes. Although these patients more frequently present with high-intermediate risk PE, their short-term prognosis is excellent with conventional anticoagulation. Well-designed studies are needed to understand why these patients more often present right ventricular dysfunction and myocardial damage in the acute phase of the PE.
Conflict of InterestThe authors state that they have no conflict of interest.
Please cite this article as: Abellás M, Menéndez A, Morillo R, Jara-Palomares L, Barrios D, Nieto R, et al. Características clínicas y pronóstico de la tromboembolia pulmonar secundaria al síndrome de clase turista. Arch Bronconeumol. 2017;53:495–500.