The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19) was first reported in Wuhan (China) on December 31, 2019.1 When the first patients infected with SARS-CoV-2 were analyzed, the genomic sequences of the single-stranded RNA virus were almost identical.2 However, as the pandemic has evolved, so has the virus genome.3
The SARS-CoV-2 Omicron variant (B.1.1.529) was first identified in November 2021. On November 26, 2021, the WHO defined the strain as a variant of concern and named it Omicron. This new variant is associated with high transmissibility leading to high infectivity and probably increased reinfection rates.4 It is currently being proven that cases of severe COVID-19 are less frequent than with previous variants. For this reason, both governmental and scientific bodies are beginning to consider the possibility that in the more or less near future, SARS-CoV-2 infection should begin to be managed as a common endemic respiratory viral disease.5,6
On November 29, the first case of infection by the Omicron variant was confirmed in Spain. According to data provided by the Center for Coordination of Alerts and Health Emergencies under the Ministry of Health of Spain, the Delta variant of the SARS-CoV-2 virus was the predominant one in our country until December 2021. After that time, it was displaced by the Omicron variant. In random sampling by specific PCR from 13 Spanish Autonomous Communities, carried out in the epidemiological week 52 of the year 2021, the proportion of infections by Omicron ranged from 48.7% (Murcia) to 93.8% (Madrid) of the total number of samples analyzed.7
A multicenter observational descriptive study was conducted in 5 secondary and tertiary hospitals of Madrid (Spain) between December 27, 2021, and January 2, 2022. Consecutive patients aged 0–16 attending the corresponding Pediatric Emergency Departments, with a positive result in the real-time polymerase chain reaction test (RT-PCR) or antigenic test to detect SARS-CoV-2 in the nasopharyngeal sample were included.
Quantitative variables were expressed by central tendency and dispersion [mean and standard deviation (± SD)]; absolute frequencies and percentages measured qualitative variables. Comparison between categorical variables was performed using the χ2 test or Fisher's exact test. A p value of <0.05 was considered statistically significant.
Seven hundred seventy-four patients were diagnosed with SARS-CoV-2 infection during the study period. Overall, 68.9% reported having been in contact with a confirmed case of COVID-19. The mean age was 6.3±4.7 years. Males accounted for 52.2% and females for 47.8%.
The most frequent clinical manifestation was fever (62.8%), with a mean duration time of 25.2±39.9h. Temperatures ≥39°C were reported in 14.3% of cases, with children aged 1–4 years presenting the highest temperatures in a higher proportion (21.8%; p=0.016) (Table 1).
Epidemiological and clinical characteristics, n(%).
| Age groups | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All cases(n=774) | <1 year(n=169) | 1-4 years(n=160) | 5-9 years(n=222) | 10-14 years(n=205) | 15-20 years(n=18) | p value |
| Sex | |||||||
| Male | 404 (52.2) | 91 (53.8) | 81 (50.6) | 128 (57.6) | 102 (49.7) | 2 (11.1) | 0.004 |
| Female | 370 (47.8) | 78 (46.1) | 79 (49.3) | 94 (42.3) | 103 (50.2) | 16 (88.8) | |
| Presenting features | |||||||
| Fever | 486 (62.8) | 120 (71) | 113 (70.6) | 126 (56.7) | 119 (58) | 8 (44.4) | 0.001 |
| ≥39°C | 111 (14.3) | 26 (15.3) | 35 (21.8) | 22 (9.9) | 25 (12.1) | 3 (16.6) | 0.016 |
| Respiratory symptoms | |||||||
| Cough | 459 (59.3) | 103 (60.9) | 106 (66.2) | 118 (53.1) | 121 (59) | 11 (61.1) | 0.141 |
| Runny nose | 414 (53.5) | 102 (60.3) | 98 (61.2) | 96 (43.2) | 109 (53.1) | 9 (50) | 0.002 |
| Difficulty breathing | 0.297 | ||||||
| No difficulty breathing | 756 (97.7) | 161 (95.2) | 156 (97.5) | 219 (98.6) | 202 (98.5) | 18 (100) | |
| Bronchiolitis/Bronchospasm | 11 (1.4) | 6 (3.5) | 3 (1.8) | 1 (0.5) | 1 (0.4) | 0 (0) | |
| Laryngitis | 4 (0.5) | 1 (0.5) | 1 (0.6) | 0 (0) | 2 (0.9) | 0 (0) | |
| Gastrointestinal symptoms | |||||||
| Vomiting | 119 (15.4) | 28 (16.5) | 24 (15) | 40 (18) | 27 (13.1) | 0 (0) | 0.246 |
| Diarrhea | 51 (6.6) | 20 (11.8) | 12 (7.5) | 9 (4) | 9 (4.3) | 1 (5.5) | 0.019 |
| Abdominal pain | 54 (7) | 1 (0.5) | 11 (6.8) | 29 (13) | 12 (5.8) | 1 (5.5) | 0.000 |
| Otorhinolaryngological symptoms | |||||||
| Sore throat | 127 (16.4) | 5 (2.9) | 17 (10.6) | 32 (14.4) | 63 (30.7) | 10 (55.5) | 0.000 |
| Otalgia | 16 (2.1) | 0 (0) | 7 (4.3) | 5 (2.2) | 3 (1.4) | 1 (5.5) | 0.055 |
| Neurological manifestations | |||||||
| Headache | 133 (17.1) | 0 (0) | 14 (8.7) | 54 (24.3) | 59 (28.7) | 6 (33.3) | 0.000 |
| Seizures | 3 (0.4) | 0 (0) | 2 (1.8) | 1 (0.5) | 0 (0) | 0 (0) | 0.327 |
| Anosmia | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) | 2 (0.9) | 0 (0) | 0.234 |
| Mucocutaneous Manifestations | |||||||
| Skin rash | 9 (1.2) | 2 (1.1) | 5 (3.1) | 0 (0) | 2 (0.9) | 0 (0) | 0.083 |
| Conjunctivitis | 6 (0.8) | 2 (1.1) | 1 (0.6) | 1 (0.4) | 2 (0.9) | 0 (0) | 0.915 |
| Others | |||||||
| Myalgia | 19 (2.4) | 0 (0) | 5 (3.1) | 1 (0.4) | 11 (5.3) | 2 (11.1) | 0.001 |
| Thoracic pain | 10 (1.2) | 0 (0) | 1 (0.6) | 4 (1.8) | 4 (1.9) | 1 (5.5) | 0.356 |
| Arthralgia | 2 (0.3) | 0 (0) | 1 (0.6) | 0 (0) | 1 (0.4) | 0 (0) | 0.678 |
Respiratory symptoms were predominant in all cases, regardless of age. The presence of cough and nasal discharge was observed in 59.3% and 53.5% of cases, respectively, and these percentages were very similar in all age ranges. Less than 3% of the cases presented with respiratory distress, mainly low respiratory distress, especially among children under 1. During their stay, chest X-rays were requested in 9 cases, 7 of which in patients who were hospitalized. In 6/9 cases (66.6%), the X-ray was considered abnormal (3 cases of interstitial infiltrates and 3 of consolidation).
Among gastrointestinal symptoms, the most frequent was vomiting (15.4%). Diarrhoea and abdominal pain were more frequent among children under 1-year-old (11.8%; p=0.019) and 5–9 years old (13%; p<0.001), respectively. Slightly more than half of the patients over 15 years of age (55.5%; p<0.001) reported sore throat. Headache (33.3%; p<0.001) and myalgia (11.1%; p=0.001) were also more frequent in this age group. Only three patients had seizures at the time of diagnosis.
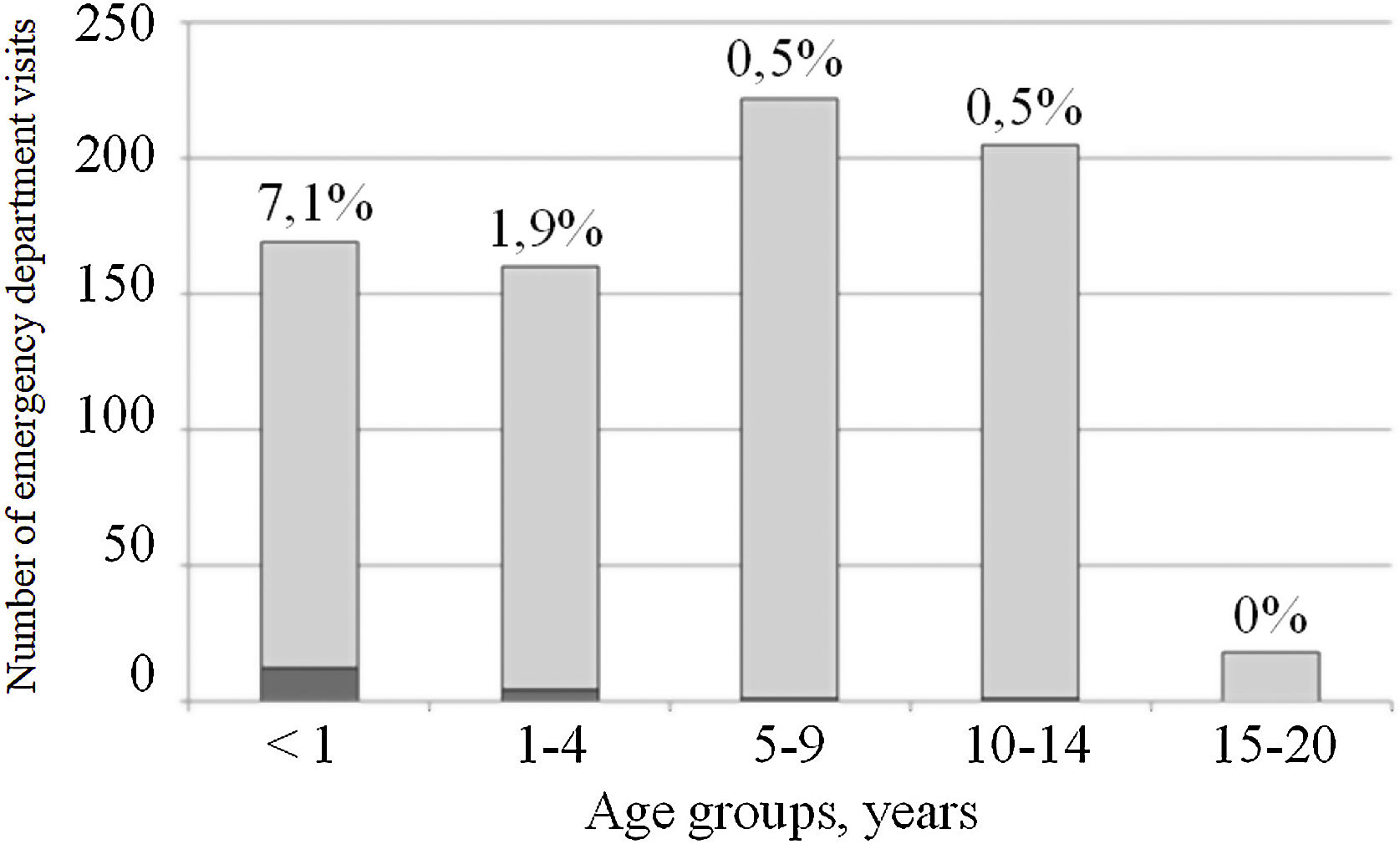
A total of 17/774 patients were hospitalized (2.3%). The rate of hospitalization was inversely proportional to the age of the cases, with the maximum rate observed being 7.1% among those under 1 year of age (Fig. 1). The main reason for admission was respiratory distress (8/17; 47%): 7 patients were admitted with a bronchiolitis diagnosis and one with severe laryngitis. In 3/17 (17.6%), the cause of hospitalization was the patients’ underlying medical conditions: one case of short bowel and two patients with hemato-oncologic pathology. In all of them, the initial reason for consultation was fever. The other reasons for admission were: 2 cases in newborns less than 1-month-old, a 2-month-old infant admitted for a 4-day persistent fever associated with rash, and a 7-year-old child admitted progressive hypoxia. Finally, in one case, the diagnosis of SARS-CoV-2 infection was done by screening for admission for diabetes debut.
Since its identification, the Omicron variant of the SARS-CoV-2 virus has shown a more rapid spread than previous variants.8 Omicron is characterized by the high number of mutations identified in its genome,9 that appear to be responsible for its high capacity to cause reinfection and its partial resistance to existing vaccines.10,11 These characteristics have enabled it to spread rapidly throughout the world,12 leading to higher levels of COVID-19 incidence than in previous pandemic periods. However, as we have also seen in our series, indicators of disease severity are lower compared to previous pandemic peaks.13,14
As with previous variants,15 fever and respiratory symptoms are the predominant symptoms of Omicron infection. However, some authors have begun to report seizures associated with infection by this variant.16 In the study by Cloete et al.17 conducted in the pediatric population of the Gauteng province in South Africa, the origin of the Omicron wave, 20% of hospitalizations were for febrile seizures.
Our hospitalization rate was 2.3%, mainly concentrated in children under 4 years of age. A multicenter study in 14 North American states18 analyzed the evolution of hospitalizations due to COVID-19, specifically in children in this age range, during the period of the predominance of Omicron; the weekly hospitalization rates were approximately five times lower than those during the period of the predominance of Delta variant.
The main reason for the hospitalization of our patients was the presence of respiratory distress. Most were diagnosed with bronchiolitis and, in one case, with laryngitis; both conditions have been previously described in the context of SARS-CoV-2 infection and are associated explicitly with the Omicron variant.19 In our series, only in one case was the diagnosis of COVID-19 made by screeinig at admission for other condition different than COVID-19. Other studies have found up to 63% of the incidental diagnosis of COVID-19 in diagnostic tests prior to hospitalization.20
Our study has some limitations: the diagnostic cases of COVID-19 analyzed were assumed to be caused by the Omicron variant based on the epidemiological analyses carried out by the Community of Madrid, which reported that Omicron was the predominant circulating variant in the study period. The variables analyzed did not include the vaccination status of the children, a factor that can undoubtedly condition the severity of the disease. However, according to data provided by the Spanish Ministry of Health, by the end of January 2022, 90.8% of the population over 12 years of age had a complete vaccination schedule. As of December 15, 2021, vaccination of children between 5 and 11 years of age was initiated.
In conclusion, Omicron variant infection in pediatric patients causes mild symptoms and is associated with a low hospitalization rate, especially in children who have not yet received SARS-CoV-2 vaccination.
Authors’ contributionsConception and design of the study: MAM. Acquisition of data: BGC, CMDR, JAR, JTRA, ICC, LST, MAGH, MAM, MDC, and MJP. Analysis and interpretation of data: JRD. All authors contributed in drafting the article.
FundingThe present investigation has not received specific support from public sector agencies, the commercial sector, or non-profit organizations.
Conflict of interestThe authors declare no conflicts of interest.