Theophylline has been shown to improve respiratory function and oxygenation in patients with chronic obstruction pulmonary disease (COPD). However, the impact of theophylline on mortality in COPD patients has not been not sufficiently evaluated.
MethodTwo investigators independently searched for eligible articles in 4 databases. The eligibility criterion for this meta-analysis was an original research article that provided a hazard ratio for theophylline for all-cause mortality of COPD patients. Both randomized controlled trials and observational studies were accepted. After we confirmed no substantial heterogeneity (I2<50%), the fixed-model method with generic inverse variance was used for meta-analysis to estimate the pooled hazard ratio.
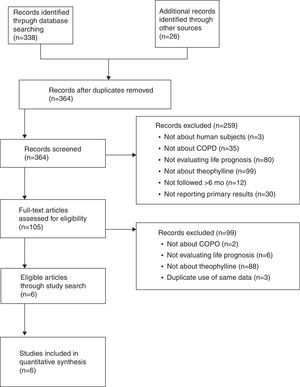
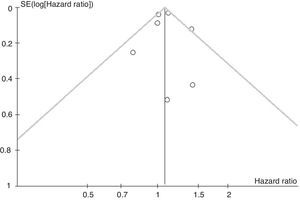
ResultsWe screened 364 potentially eligible articles. Of the 364 articles, 259 were excluded on the basis of title and abstract, and 99 were excluded after examination of the full text. Our final analysis included 6 observational studies and no randomized controlled trials. One study reported 2 cohorts. The number of patients in each cohort ranged from 47 to 46403. Heterogeneity (I2=42%, P=.11) and publication bias (Begg's test r=0.21, P=.662) were not substantial. Fixed-model meta-analysis yielded a pooled hazard ratio for theophylline for all-cause death of 1.07 (95% confidence interval: 1.02–1.13, P=.003).
ConclusionThis meta-analysis of 7 observational cohorts suggests that theophylline slightly increases all-cause death in COPD patients.
Se ha demostrado que la teofilina mejora la función respiratoria y la oxigenación en pacientes con enfermedad pulmonar obstructiva crónica (EPOC). Sin embargo, no está suficientemente evaluado el impacto de la teofilina sobre la mortalidad de los pacientes con EPOC.
MétodoDos investigadores buscaron de forma independiente artículos elegibles en 4 bases de datos. Los artículos seleccionados para el presente metaanálisis debían ser artículos de investigación originales que proporcionaran el cociente de riesgos instantáneos (HR) de la mortalidad por cualquier causa en pacientes con EPOC tratados con teofilina. Se permitió incluir tanto ensayos controlados aleatorizados como estudios observacionales. Después de confirmar que la heterogeneidad no era significativa (I2<50%), para estimar el cociente de riesgos instantáneos del metaanálisis se empleó un modelo fijo con un método de varianza inversa genérica.
ResultadosSe seleccionaron 364 artículos potencialmente elegibles. De los 364 artículos, 259 fueron excluidos basándose en el título y el resumen, y 99 fueron excluidos después de considerar sus textos completos. Finalmente, nuestro análisis incluyó 6 estudios observacionales y ningún ensayo controlado aleatorizado. Un estudio estaba realizado con 2 cohortes. El número de pacientes en cada cohorte varió de 47 a 46.403. La heterogeneidad (I2=42%, p=0,11) y el sesgo de publicación (r=0,21, p=0,662 en la prueba de Begg) no fueron significativos. El metaanálisis del modelo fijo arrojó un cociente de riesgos instantáneos combinado de mortalidad por cualquier causa con teofilina de 1,07 (intervalo de confianza del 95%: 1,2-1,13, p=0,003).
ConclusiónEl presente metaanálisis de 7 cohortes observacionales sugiere que la teofilina aumenta ligeramente la mortalidad por cualquier causa de los pacientes con EPOC.
Chronic obstructive pulmonary disease (COPD), now the fourth leading cause of death worldwide, is a systemic disease characterized by chronic airflow limitation.1 As such, bronchodilator medications, namely theophylline, long-acting muscarinic antagonists (LAMA), and long-acting beta-agonists (LABA), are understandably used as key medications for treating stable COPD patients.1 Among these 3 subclasses of bronchodilators, LAMAs and LABAs have repeatedly been reported as safe and effective in randomized controlled trials (RCT) and large observational studies.1–7 LAMAs and LABAs are, therefore, now regarded as first-line medications for stable COPD.1 The efficacy of theophylline, meanwhile, has been shown in many observational studies and relatively small RCTs.8–18 These studies generally indicated that theophylline improves values in respiratory function tests and arterial blood gas analyses, but that it may negatively affect the risk of exacerbation and side effects.8–18 Even though clinicians are usually interested in patient survival, death was not used as the main outcome. Other surrogate outcomes such as oxygenation or respiratory function were used instead, because an insufficient number of deaths was observed during the follow-up period. However, these surrogate markers do not always reflect the mortality of respiratory disease with airflow obstruction. For example, short-acting beta-agonists improve airflow obstruction, dyspnea, and quality of life in bronchial asthma patients, but regular use of these agents increases the number of deaths from bronchial asthma.19,20 Accordingly, the impact of theophylline on mortality in COPD patients is still an important research question.
Some observational studies have investigated the impact of theophylline treatment on mortality in COPD patients.21–26 However, the results of these studies varies widely. The association between theophylline and mortality risk is still an important matter for all clinicians, as theophylline is a common bronchodilator that has been used for decades.1,27 The aim of this systematic review and meta-analysis, then, was to evaluate the impact of theophylline on all-cause death in COPD patients.
MethodsStudy Search CriteriaRequirements for institutional review board approval and informed consent were waived for this study because of the anonymous nature of the data and the fact that it was a review study.
The eligibility criterion for the meta-analysis was an original research article that provided a hazard ratio (HR) for theophylline for all-cause death in COPD patients. Both RCTs and observational studies were accepted. For observational studies, adjusted HR was preferred to non-adjusted HR. Follow-up duration had to be >6 months. Duplicate use of the same data was excluded.
Two investigators independently searched for eligible articles using the MEDLINE, Web of Science, EMBASE, and Cochrane Databases as of January 2014. The search formula for MEDLINE was ((“COPD”[title] OR “chronic obstructive pulmonary disease”[title] OR “chronic obstructive airway disease”[title] OR “emphysema”[title] OR “chronic bronchitis”[title] OR “chronic airflow obstruction”[title]) OR ((“COPD” OR “chronic obstructive pulmonary disease” OR “chronic obstructive airway disease” OR “emphysema” OR “chronic bronchitis” OR “chronic airflow obstruction”) and (“theophylline” OR “xanthine” OR “theophyllines” OR “xanthines” OR “aminophylline” OR “diprophylline” OR “proxyphylline” OR “diprophylline”))) and (“mortality” OR “prognosis” OR “death” OR “mortalities” OR “prognoses” OR “deaths”) and (“hazard ratio” OR “HR” OR “hazard ratios”).
The quality of eligible studies was evaluated using a scale comprising 4 subscales with a maximum of 2 points for each subscale.28 The subscales were cohort entry, exposure definition, outcome, and cofounding assessment. The scores ranged from 0 to 8, where a higher score signified better quality28
StatisticsWe extracted HR for all-cause mortality in each study. HR and its 95% confidence interval [95% confidence interval (CI)] had to be clearly demonstrated in a text, a table, or a figure in each original study. Additionally, data for number of patients, concomitant administration of beta-stimulants and anti-cholinergic agents, and covariables adjusted for the Cox model were extracted.
The fixed-model method with generic inverse variance was used for meta-analysis to estimate pooled value, after no significant heterogeneity was confirmed (I2<50%).29–31 Heterogeneity among the original studies was evaluated with (i) the Chi-square distribution test with a rejection region of P=.1, and (ii) the I2 statistics test whereby I2=0% indicates no heterogeneity, I2=25% indicates mild heterogeneity, I2=50% indicates moderate heterogeneity, I2=75% indicates strong heterogeneity.30,31 Publication bias was evaluated with a funnel plot, and with Begg's test using the Spearman's rank correlation test with a rejection region of P=.1.32 All analyses were performed using Excel Toukei 2012 (SSRI, Tokyo. Japan), and Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK).
ResultsStudy Search ResultsWe screened 364 potentially eligible articles. Of the 364 articles, 259 were excluded on the basis of title and abstract, 99 were excluded after the full text was examined. Lee reported 4 articles analyzing overlapping data reporting risk of death by treatment regimen using HR,25,33–35 of which we included 1 article that focused on impact of theophylline on death25 and excluded the others33–35 due to duplicate use of the same data. Six studies were finally included in our analysis (Fig. 1).21–26 These 6 were published between 2003 and 2011. Macie grouped COPD cases into patients aged under 65, and those aged 65 or older.24 Lee reported some HRs for theophylline for all-cause death.25 One HR was calculated with a time-varying exposure analysis in which patients were treated with variety of medications. Each of the other HRs in Lee's study was calculated with specific regimen. Duplicate data was found in these calculations; for this study, therefore, we used HR by time-varying exposure analysis.
The main meta-analysis included 7 cohorts. The number of patients in each cohort ranged from 47 to 46403, for a total of 60692 COPD patients (Table 1). The quality score of Lee's study was 6 out of 8. The quality scores of the other studies included were 7 out of 8. Those scores meant that the quality of these observational studies was generally good (Table 1). Adjusted co-variables in the Cox model are also summarized in Table 1. No study provided succinct data concerning theophylline dose or blood levels.
Summary of Original Studies Included for Meta-analysis.
| Study | Design SQ | Patients | Follow-up duration | Co-variables adjusted for Cox model | Hazard ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Recruitment, definition | No. | Inhaled beta-agonist | Inhaled anti-cholinergic | |||||
| Fan (2003)21 | Pro 7 | Outpatients on inhaled corticosteroids. ICD-9 | 8052 | 88.0% (SABA) | 72.8% (not specified) | 544 days | Pulmonary medication, age, hospital site, prior COPD visits, comorbidity, distance to hospital | 1.00 (0.84–1.20) |
| Nizet (2005)22 | Pro 7 | Hypercapnic, ATS | 47 | Not available | Not available | 3.8 years | Age, sex, comorbidity, diuretics | 1.10 (0.40–3.10) |
| Gudmundsson (2006)23 | Pro 7 | Post exacerbation | 416 | 39.6% (LABA) | 35.1% (Ipratropium) | 2 years | Age, sex, center, smoking, FEV1, previous hospitalization, SGRQ, comorbidity, pulmonary medication, long-term oxygen | 0.79 (0.48–1.30) |
| Macie (2006)24 <65 years old; ≥65 years old | Ret 7 | Post admission, ICD-9 | 965 4022 | 54.9% (not specified) 61.2% (not specified) | 30.6% (Ipratropium) 44.7% (Ipratropium) | 365 days | Selected covariates | 1.41 (0.60–3.34) 1.40 (1.10–1.79) |
| Lee (2009)25 | Ret 6 | Veterans, ICD-9 | 7840 | 48.2% (LABA) | 88.2% (Ipratropium) | 2.5 years | Baseline propensity to receive theophylline, exacerbation, age | 1.23 (1.09–1.39) |
| Gershon (2011)26 | Ret 7 | Population based | 46403 | 38.4% (LABA) | 61.6% (LAMA) | 5.5 years | Age, sex, resident in a long-term care facility, income, place to live, COPD duration, previous spirometry, physician visits, specialist visit, influenza vaccination, lung volume reduction surgery, COPD medications, coronary medications, comorbidities, recent hospitalization/emergency visit. | 1.01 (0.93–1.10) |
SQ, study quality, wherein 8 is the best score; Pro, prospective cohort study; Ret, retrospective cohort study; ICD-9, International Classification of Diseases, 9th revision; ATS, American Thoracic Society. Macie grouped cases into cases aged under 65, and cases aged 65 or higher. SABA, short-acting beta agonist; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; SGRQ, total score of St. George Respiratory Questionnaire.
The proportion of patients prescribed other bronchodilators, such as beta-agonists and anticholinergics, are shown in Table 1. There was no specific association between HR for all-cause death and prescription of these bronchodilators in any of the studies (Table 1)
Meta-analysisEach of the 7 cohorts presented an HR of 0.79–1.41, suggesting weak or moderate heterogeneity without significance (I2=42%, P=.11). Fixed-model meta-analysis yielded a pooled HR for theophylline for all-cause death of 1.07 (95% CI 1.02–1.13, P=.003) (Fig. 2). Neither a funnel plot nor Begg's test (r=0.21, P=.662) suggested the existence of publication bias (Fig. 3).
We conducted a systematic review and meta-analysis of observational studies to evaluate the impact of theophylline on mortality in COPD patients. Fixed-model meta-analysis yielded a pooled HR for theophylline for all-cause death of 1.07 (95% CI 1.02–1.13, P=.003) (Fig. 2). This suggests that theophylline may be associated with a slight increase in all-cause death in COPD subjects.
Pharmacologic therapy for COPD is used to reduce symptoms, frequency of exacerbations, and death. To date, few of the existing agents have been sufficiently shown to modify long-term deterioration in lung function.1 Bronchodilators increase forced expiratory volume in one second, usually by altering the smooth muscle tone of the airway. Among bronchodilators, theophylline is less effective and less well tolerated than LAMAs and LABAs, and is not recommended as a first-choice medication. Toxicity is dose-related and the therapeutic index is small. Most of the benefit occurs only when near-toxic doses are used.1 Furthermore, it theoretically more easily causes systemic side effects than LAMAs and LABAs, as it has to be administrated systematically.1
The therapeutic effects of theophylline are still being debated. It acts as a non-selective phosphodiesterase inhibitor, but also has non-bronchodilator action, i.e. improvement of inspiratory muscle function and an anti-inflammatory effect. Zhou conducted a double-blind, parallel-group, placebo-controlled RCT in 110 subjects to evaluate the therapeutic effect of low-dose treatment (100mg twice daily).36 This low dose of theophylline was not considered as a bronchodilator, but as an anti-inflammatory agent.37 In the study, even though low-dose theophylline did not improve post-bronchodilator lung function, theophylline-treated subjects showed improved quality of life, and lower rates of exacerbation and hospitalization.36 Considering recent emerging consensus on the link between exacerbations and mortality, low-dose theophylline may hint at a new treatment strategy.
Limitations of the current study should be discussed. The current meta-analysis included limited numbers of cohorts. In addition, all studies included in the meta-analysis were observational studies, not RCT. However, meta-analysis using non-RCT articles has recently become widely accepted.38 Another limitation is that no original article provided sufficient data on theophylline dosing and blood levels. Nonetheless, we still believe that the results of this analysis are reliable, given that the observational studies included showed neither heterogeneity nor publication bias.
In conclusion, current guidelines supporting the chronic use of theophylline for COPD cases are based on a few studies focusing on respiratory function tests and arterial blood gas analyses.8,9 No RCT has evaluated the impact of theophylline on all-cause death. This meta-analysis of 7 observational studies suggests that theophylline slightly increases all-cause death in COPD patients with a pooled HR for theophylline for all-cause death of 1.07 (95% CI 1.02–1.13). These results should be taken into account when deciding on the use of theophylline in some COPD patients.
Financial StatementNo support in the form of grants, gifts, equipment, and/or drugs was received.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Horita N, Miyazawa N, Kojima R, Inoue M, Ishigatsubo Y, Kaneko T. Uso crónico de teofilina y mortalidad en la enfermedad pulmonar obstructiva crónica: un metaanálisis. Arch Bronconeumol. 2016;52:233–238.