Systemic air embolism (SAE) is the rarest and most unpredictable complication of CT-guided lung biopsy, with an estimated incidence of 0.061–0.21%.1 It may occur due to the formation of a transient broncho-venous fistula after needle penetration of the lung or inadvertent puncture of a pulmonary vein; furthermore, air can reach the venous circulation through the pulmonary microvasculature.2–4 We report a case of cerebral air embolism after CT-guided lung biopsy, diagnosed according to the Hare et al.5 algorithm (Table 1) and treated in a hospital setting without a hyperbaric chamber. The outcome was positive, and the patient was discharged without permanent sequelae.
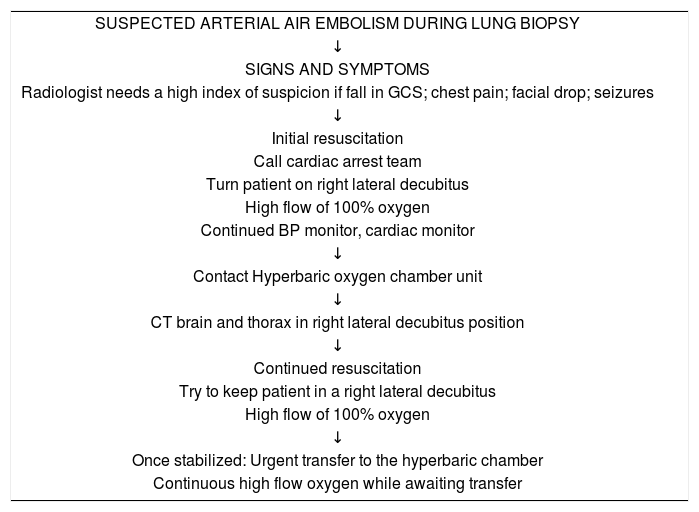
An algorithm for the recognition and early management of suspected systemic arterial air embolism post-lung biopsy (by Hare et al. Clin Radiol. 2011 Jul; 66(7) 589–596).
| SUSPECTED ARTERIAL AIR EMBOLISM DURING LUNG BIOPSY |
| ↓ |
| SIGNS AND SYMPTOMS |
| Radiologist needs a high index of suspicion if fall in GCS; chest pain; facial drop; seizures |
| ↓ |
| Initial resuscitation |
| Call cardiac arrest team |
| Turn patient on right lateral decubitus |
| High flow of 100% oxygen |
| Continued BP monitor, cardiac monitor |
| ↓ |
| Contact Hyperbaric oxygen chamber unit |
| ↓ |
| CT brain and thorax in right lateral decubitus position |
| ↓ |
| Continued resuscitation |
| Try to keep patient in a right lateral decubitus |
| High flow of 100% oxygen |
| ↓ |
| Once stabilized: Urgent transfer to the hyperbaric chamber |
| Continuous high flow oxygen while awaiting transfer |
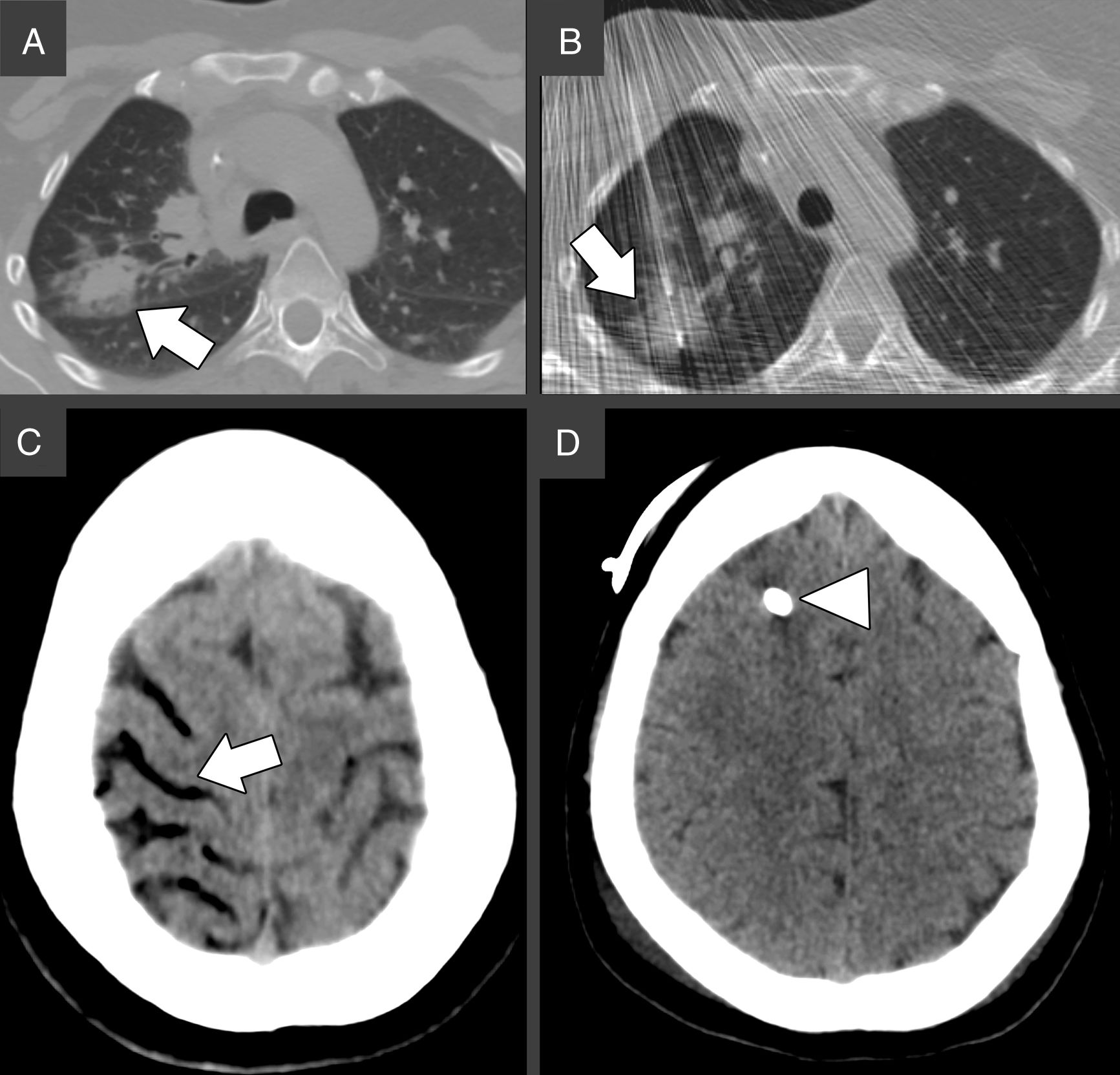
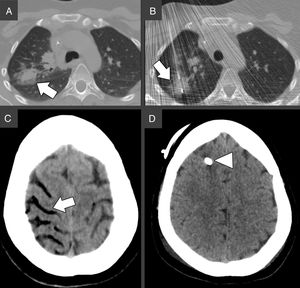
A 36 years old woman affected by Chron's Disease and Hodgkin Lymphoma, treated with allogeneic transplant, came to our attention for CT-guided biopsy of an FDG/PET-positive pulmonary nodule located in the right upper lobe, suspicious for lymphoma relapse (Fig. 1A).
(A) Chest CT prior biopsy. The white arrow indicates the target lesion in the right upper lobe, in close proximity to the main fissure. (B) Chest CT during the biopsy. An 18-gauge Tru-Cut needle is successfully advanced through the target nodule under continuous CT-fluoroscopy guidance, as indicated by the white arrow. (C) Brain CT 19min after the biopsy. The white arrows indicate air in the right superior frontal, precentral, central (A) and superior temporal (B) sulci, consistent with cerebral venous air embolism. (D) Brain CT 3 days after biopsy showing complete resorption of the air bubbles. The white arrowhead indicates the external ventricular derivation.
After obtaining the informed consent and under local anesthesia, an 18-gauge Tru-Cut core biopsy needle (Argon Medical Devices, USA) was inserted under fluoroscopy-CT guidance through the right pectoral muscles. A specimen of the lesion was collected in a single shot (Fig. 1B). Immediately after needle removal, the patient experienced hemoptysis and had a tonic–clonic seizure. According to the abovementioned algorithm, the cardiac arrest team was called, 100% oxygen supply was started and both chest and brain CT were performed, 19min after lung puncture. The brain CT showed mild cerebral edema and the presence of bilateral intracranial air, mainly located in the right frontal and parietal lobes, suggesting SAE (Fig. 1C). The patient was then moved to the right lateral decubitus and high flow oxygen was continued during its transfer to the intensive care unit. Neurological assessment scored 8 points in the Glasgow Coma Scale (Eyes=2, Verbal=2, Motor=4), which prompted the placement of an external ventricular derivation to control the intracranial pressure. A drug-induced coma was maintained for 48h. Upon awakening, the patient's vitals normalized, and the neurological deficits improved. The brain CT performed 3 days after the biopsy, showed complete resorption of the cerebral air bubbles and normal permeability of the main intracranial venous sinuses. She was discharged 10 days later, after complete recovery.
The pulmonary biopsy was positive for secondary localization of lymphoma and the patient died 8 months later due to graft-versus-host disease.
In this report, we showed how the use of the Hare et al. algorithm enabled a rapid diagnosis and quickened the decision-making treatment process. Our experience is limited to this single case; it is, however, important by virtue of cerebral SAE rarity and its life-threatening characteristics. In fact, most SAE reports describe cardiac and respiratory symptoms,6 whereas only a few describe neurological manifestations. As reported by Kim et al., even simple needle insertion into the chest wall can cause air to flow through the pulmonary venous circulation thus, piercing the lung parenchyma may not be the only maneuver at risk for SAE.7
Our treatment course deviated from the recommendations previously described due to the absence of a hyperbaric chamber in our hospital and to the placement of a ventricular derivation, never reported before to treat SAE (Fig. 1D). This choice proved successful. It is in fact known that many forms of acute brain injury benefit from cerebrospinal fluid diversion and the continuous monitoring of intracranial pressure provided by the insertion of an external ventricular derivation which is one of the lifesaving procedures in the neurologic intensive care unit.8
In conclusion, physicians performing lung biopsies should be aware of the unpredictable manifestations of SAE and mindful of the usefulness of an emergency algorithm for its management.
The authors wish to thank all the colleagues who contributed to the care of this patient, particularly Dr. Federico Barra for the brilliant management of the acute phase and Dr. Simona Marcheselli for the neurological assessment throughout the observation.