The development of biologics has led in recent years to a breakthrough in the treatment of chronic inflammatory joint disease, especially rheumatoid arthritis (RA). This group of drugs includes tumor necrosis factor (TNFα) inhibitors, which have proven efficacy in the treatment of this disease.1 TNFα inhibitors are generally well tolerated and show an acceptable safety profile. However, the inhibition of TNFα, a cytokine that plays an essential role in inflammation and response to infection, has been associated with an increased likelihood of infectious complications.2 Reports have also emerged in the last 10 years of non-infectious systemic and pulmonary side effects, including malignancies3 and secondary autoimmune disorders.4,5
We report the case of a 62-year-old woman, former smoker of 15 pack-years, with a 10-year history of palindromic rheumatism that progressed to RA with bone erosion. She received treatment with various disease-modifying antirheumatic drugs (DMARD), such as hydroxychloroquine sulfate, methotrexate, and gold salts, together with NSAIDs (indomethacin and naproxen) and low-dose prednisone, depending on her symptoms, at different disease stages.
Between May 2010 and March 2012, due to a lack of response to DMARDs, she received biological treatment with anti-TNFα (etanercept) for the first time, with improvement of her joint symptoms. In March 2016, when she was receiving treatment with methotrexate (20mg/week) for a new flare, etanercept was reintroduced (50mg/week), again resulting in clinical improvement.
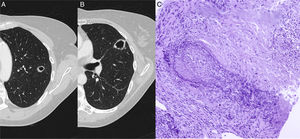
In a radiological follow-up of the disease, when the patient was asymptomatic, a chest X-ray and computed tomography (CT) were obtained, showing 2 solid cavitating nodules, one measuring 15mm in the apicoposterior segment of the left upper lobe (Fig. 1A) and the other 24mm in the upper segment of the lingula (Fig. 1B).
(A) Chest CT showing a cavitating nodular lesion in the left upper lobe and (B) another in the upper segment of the lingula. (C) Staining of elastic fibers to highlight the irregular destruction of the arterial wall by the inflammatory process. Necrosis is seen in the upper right corner. Orcein 200×.
Laboratory tests, including complete blood count, coagulation, blood biochemistry, and tumor markers, were normal. CRP and ESR were 0.42ng/dl and 27mm/h, respectively. Autoimmune tests showed elevated cyclic citrullinated peptide antibodies (381IU) and rheumatoid factor (200IU), with values similar to previous determinations. Basic urine profile showed no significant changes. Serum anti-neutrophil cytoplasmic antibodies determined by immunofluorescence were negative, as was ELISPOT-TB.
Fiberoptic bronchoscopy was performed, showing no endobronchial abnormalities, and microbiological and cytological studies of the bronchial aspirate and bronchoalveolar lavage were negative.
Positron emission tomography was also performed, showing annular, irregular 18F-fluorodeoxyglucose uptake limited to the pulmonary nodules, with a SUVmax of 2.9 in the lesion located in the left upper lobe and 3.1 in the lesion located in the lingula.
A pulmonary biopsy was obtained from the lingular lesion using CT-guided Tru-Cut. Histopathological study showed a granulomatous inflammatory lesion associated with vasculitis consistent with granulomatosis with polyangiitis (formerly known as Wegener's granulomatosis) (Fig. 1C).
In view of the diagnostic suspicion of granulomatosis with polyangiitis associated with the administration of etanercept, this drug was discontinued and treatment began with prednisone 1mg/kg/day in a tapering regimen, and the background biological treatment was replaced by tocilizumab (8mg/kg/month). Chest CT 3 months later showed resolution of the lesions.
The exclusion of other pulmonary complications, the temporal association with the administration of etanercept, and the rapid and complete resolution of the pulmonary lesions after its withdrawal and subsequent treatment with corticosteroids confirm the diagnosis of vasculitis associated with etanercept.
The spectrum of pulmonary manifestations of RA is wide and includes involvement of the parenchyma (interstitial lung disease, pulmonary nodules), airway (bronchiolitis obliterans, bronchiectasis), pleura (effusion, bronchopleural fistula, pneumothorax), and the pulmonary vasculature (pulmonary hypertension, thromboembolic disease), that may precede joint symptoms in 10%–20% of cases.6 This case shows that the differential diagnosis must also include side effects of immunomodulatory therapy (toxicity, infection) used in the treatment of these patients.
The mechanisms causing anti-TNFα to trigger antibody formation and autoimmune processes are not fully clarified. There is evidence that treatment with anti-TNFα is associated with a higher production of antinuclear antibodies, depending on the type of anti-TNFα used. In the case of etanercept, antinuclear antibody levels range from 11% to 54%,7–9 and this has also been associated with the development of other autoimmune diseases, such as leukocytoclastic vasculitis, accelerated rheumatoid nodulosis, and vasculitis associated with anti-neutrophil cytoplasmic antibodies.10 Clinical manifestations are variable, and patients, like ours, often have no associated systemic symptoms. Vasculitis associated with anti-TNFα therapy is a rare complication, and in most cases the presentation is exclusively cutaneous11; pulmonary involvement in these patients is unusual. Ramos-Casals et al. reported a series of 233 patients with anti-TNFα-induced autoimmune diseases, including 133 cases of vasculitis. Of these, only 3 were pulmonary (3%).12 Treatment consisted of discontinuing the drug and administering corticosteroids.
In conclusion, while anti-TNFα offers clear advantages in the management of RA, these biologics should be used with caution and under close monitoring, particularly in patients with pre-existing lung disease.13 If lesions and/or pulmonary infiltrates develop during treatment with anti-TNFα the first step is to rule out infectious processes. If results are inconclusive, infiltrates are persistent, and the index of suspicion is high, invasive tests such as transbronchial biopsy, cryobiopsy, CT-guided biopsy, or even surgical biopsy are recommended in order to obtain a definitive diagnosis and to indicate the correct treatment.
Please cite this article as: Martín-de León R, Moisés J, Peris P, Agustí C, Marrades RM. Nódulos pulmonares cavitados en paciente tratada con anti-TNF. Arch Bronconeumol. 2018;54:431–432.