Systemic amyloidosis is caused by the extracellular deposition of protein in the form of fibrils, known as amyloid.1 This process causes functional impairment of the affected organs and is fatal if left untreated.
Lung involvement is relatively common, but rarely symptomatic. It can present in three ways: nodular, tracheobronchial and diffuse alveolar septal.
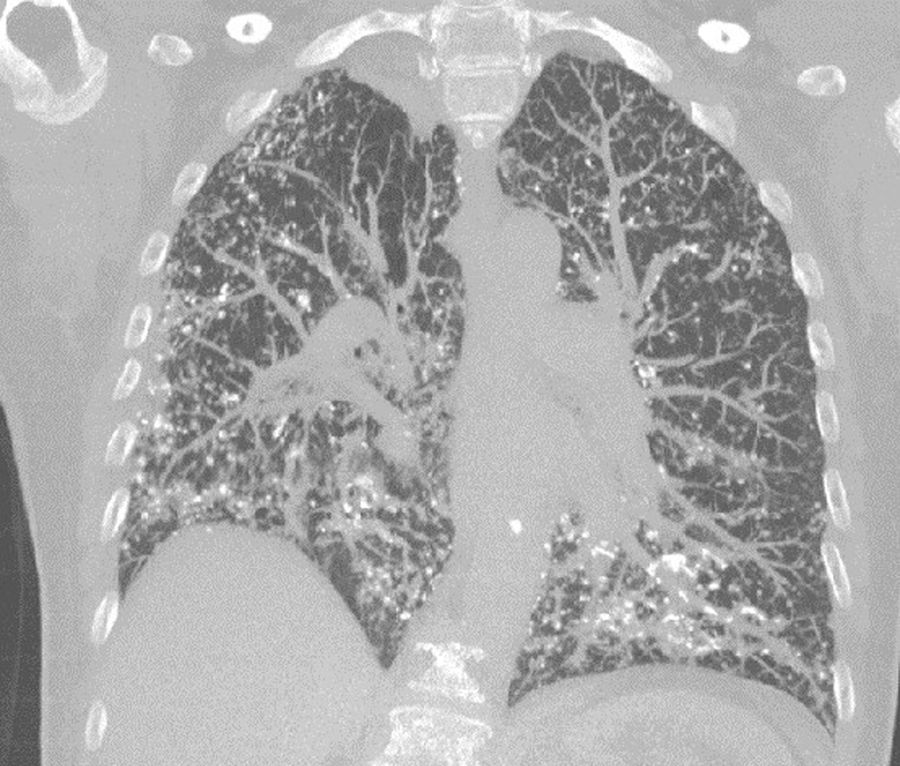
We describe below the case of a female patient, 65 years of age, history of COPD, former smoker, who reported a 6-month history of progressive dyspnea, with no other associated symptoms. On physical examination, she had dry crackles in both lung bases. Complete blood count and biochemistry were normal. An echocardiogram was performed, which was normal. Spirometry revealed a severe obstructive defect (FEV1 47%) with moderate restriction (FVC 61%). Diffusing capacity for CO was severely reduced (17%). The 6-min walk test showed a significant drop in oxygen saturation and had to be suspended due to significant dyspnea and O2 saturation of 73%. A chest computed tomography (CT) was performed that showed multiple nodular images, mostly densely calcified, with thickening of interlobular septa, mainly peripheral and in the basal segments (Fig. 1). A lung biopsy, performed by video-assisted thoracoscopy, showed the presence of nodular foci of osseous metaplasia.
Two weeks after the biopsy, the patient developed edema of the lower limbs below the knee. Urine testing showed proteinuria 3.6g/24h and hypoalbuminemia 2.8g. Nephrotic syndrome was diagnosed and a renal biopsy was performed, consistent with light-chain (AL) amyloidosis, with lambda light chain as a precursor of amyloid. Lambda chains of 723mg/L (5.7–26) were found in blood. A bone marrow biopsy showed 40% plasma cells, confirming the diagnosis of multiple myeloma.
In view of these findings, the lung biopsy was reviewed and found to be positive for red Congo and birefringence both in the blood vessel walls and the alveolar septa, confirming lung involvement in the form of pulmonary alveolar septal amyloidosis.
Treatment started with bortezomib and glucocorticoids. An echocardiogram was repeated 2 months after the diagnosis, showing an increase in refringence in the myocardium and endocardium of both ventricles, consistent with infiltrative cardiomyopathy. The patient died 1 month later due to cardiac decompensation.
Pulmonary alveolar septal amyloidosis is characterized by the deposition of amyloid in the alveolar septa and blood vessels. In general, it is a component of systemic AL amyloidosis compromise, and is most often caused by the deposit of lambda light chains. Several case series report that pulmonary alveolar septal involvement occurs in 78–90% of cases of AL amyloidosis.2,3
The clinical presentation of pulmonary alveolar septal amyloidosis is more severe than with other types of pulmonary amyloidosis, as the deposits occur in the interstitium and affect gas exchange. It presents as progressive dyspnea, not attributable to other causes. This is reflected in lung function tests that show a restrictive pattern with a reduced diffusion capacity for CO and hypoxemia on exertion.4
High-resolution CT shows reticulonodular opacities, septal thickening, and less frequently, ground glass opacification, bronchiectasis, and honeycombing.5
Histologically, amyloid may adopt an interstitial nodular pattern that can diffuse or form plaques. It can also be seen in the vascular walls. Interstitial deposits can go unnoticed. As in this case, calcifications and foci of osseous metaplasia can be observed.6
The treatment of this disease is the same as for systemic AL amyloidosis. Reducing light chain concentrations in blood may improve organ dysfunction.7 However, no data are available on the impact of treatment on pulmonary compromise.
Please cite this article as: Kevorkof GV, Oviedo E, Najo M, Camporro FA. Amiloidosis pulmonar alveolo septal calcificada como manifestación inicial de mieloma múltiple. Arch Bronconeumol. 2018;54:536–537.