Bronchiectasis (BE) is the third most common chronic inflammatory airway disease after COPD and asthma. The growing interest in this disease in the last two decades has led to an upsurge in its diagnosis but, unfortunately, the level of therapeutic scientific evidence has not increased at the same rate. This represents a major obstacle to develop guidelines based strictly, and inevitably, on the scientific information available from current systems such as the GRADE methodology. Accordingly, it is often essential to turn to the recommendations of the expert authors who drew up the various guidelines when confronting the huge variety of situations arising in day-to-day clinical practice. Nevertheless, it is encouraging to find that, despite this paucity of evidence on BE, most of the prevailing national and international guidelines are in full agreement about key aspects of the management of BE patients.
The Spanish Guidelines on BE, issued by SEPAR (2008),1 were the first of their kind in the world and drew on the experience of clinicians with over 20 years of experience in both cystic fibrosis (CF) and BE who proposed treatments that had demonstrable beneficial effects on the patients. A decade later, the new Spanish Guidelines (2018)2,3 incorporated the strict methodological system adopted by the SEPAR Scientific Committee, following the advice of a methodology specialist as well as an experienced multidisciplinary group of clinicians capable of establishing a good balance between the (sparse) scientific evidence and their own extensive experience.
One of the key clinical areas that gives rise to the most discrepancies in the various BE guidelines is the management of chronic bronchial infection (CBI) by Pseudomonas aeruginosa (PA), which has been shown to triple mortality rates in BE patients in a meta-analysis of 21 studies.4 From their its earliest version, the Spanish Guidelines have advocated an aggressive long-term antibiotic treatment for all patients in this situation, but other guidelines (e.g., the European and British Guidelines) consider that, according to the evidence available from clinical trials on macrolides or inhaled antibiotics, such treatment should be confined to patients with at least three exacerbations per year. In our view there are some circumstances that could explain this divergence of opinions and render them compatible.5,6
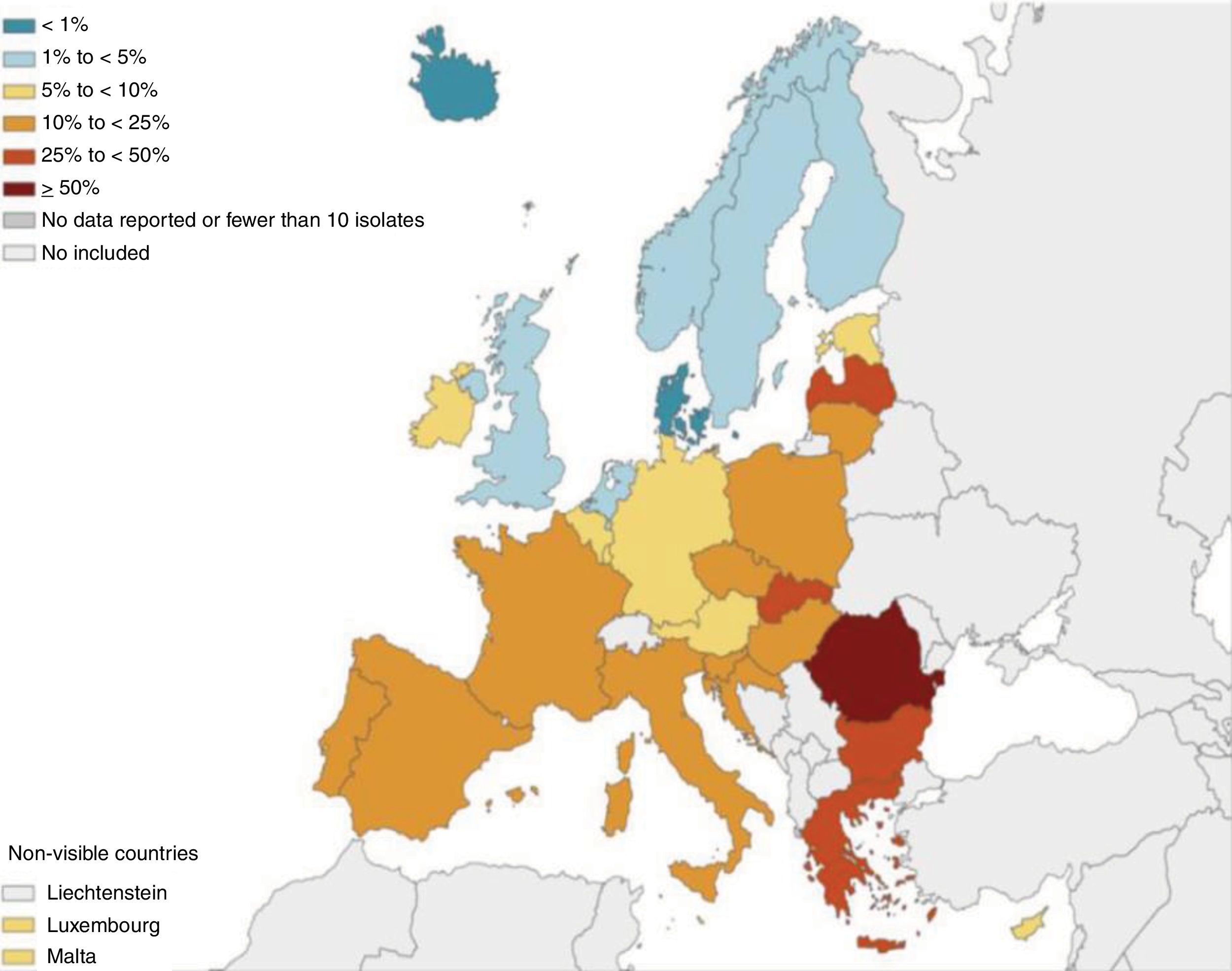
Firstly, the data from BE registries and some studies clearly show that the prevalence of PA isolates in BE patients increases going southward from the Nordic and Central European countries and is twice as high in the Mediterranean area, including Spain.7 These data have been corroborated (including CBI by PA) by the Spanish registry8 and by Latin American9 and Asian studies.10 Moreover, and even more worryingly, the degree of resistance of PA to the antibiotics most used against respiratory and systemic infection by this microorganism seems to follow a similar geographical pattern, which could limit the efficacy of oral and systemic treatments (Fig. 1).11 Should all patients with CBI by PA be treated in the same way, regardless of the local microbiological pressure? We think not, as an early aggressive treatment would halt the progress of PA and therefore reduce its detrimental effects. Simply waiting for a less favourable clinical situation in an epidemiological setting of increased resistance to antibiotics would hugely reduce any probability of controlling the chronicity of this infection.
Pseudomonas aeruginosa. Percentage (%) od Pseudomonas aeruginosa invasive isolates with combined resistance to three or more antimicrobial groups including piperacillin-tazobactam, ceftazidime, fluoroquinolones, aminoglycosides and carbapenems (EU/EUA countries, 2017).
Secondly, various international guidelines have established, on the basis of the scientific evidence, the need for aggressive treatment against CBI by PA in BE due to CF,12 even though some drugs have not proved equally effective and safe in both diseases. Would it be reasonable, faced with a lack of specific scientific evidence on the treatment of CBI by PA in BE not due to CF, to adopt therapeutic measures similar to those used to CF, in the light of the great aggressiveness of this microorganism in BE of any aetiology? We believe this to be the case.
Finally, some authors have shown that it is questionable, to say the least, to make the number of exacerbations the main endpoint of clinical trials (CTs) that evaluate the effectiveness of long-term antibiotic therapy against CBI by PA in BE. This endpoint is of limited value due to the customary inclusion in such studies of patients with a high incidence of exacerbations. Furthermore, other variables have demonstrated great prognostic value, regardless of the number or severity of exacerbations: for example, deterioration in the quality of life (a key parameter for regulatory agencies), immunocompromised, elderly or fragile patients, multiple comorbidities, reduced lung function and the severity of the symptoms, or indeed BE itself. It is therefore essential to consider the most appropriate endpoint for these CTs4,12 (maybe it should be weighted and composite). In this respect, according to the Spanish registry, more than 95% of patients with BE and CBI by PA would fulfil some of the aforementioned clinical criteria.8 Aliberti et al. came to similar conclusions in a study involving five European centres in which 93–95% of the cluster comprising patients with CBI by PA presented coughing and daily expectoration and a median of three exacerbations in the previous year.13 Does it therefore seem reasonable to treat all the aforementioned cases in a country with a high prevalence of both CBI by PA and PA resistant to antibiotics? Once again, our reply has to be affirmative.
For all these reasons, we recommend that all patients with CBI by PA and BE be treated with inhaled antibiotics, regardless of the number of exacerbations undergone (although these are usually frequent). We also recommend that this treatment be applied in countries with an environment of microbiological pressure similar to that of Spain. We are aware that such treatment is not free of costs and adverse effects and that it can sometimes be inaccessible. It therefore seems reasonable to propose a less aggressive and more selective treatment in areas without excessive microbiological pressure, as other options could potentially produce a better response.
In summary, the scarcity of therapeutic information on BE makes it necessary to find the right balance between scientific evidence, experience and local geographical factors. This gives added value to the logical and necessary coexistence of international and national guidelines, i.e., of those based more on scientific evidence and others incorporating therapeutic modifications that reflect local circumstances, so that between them a response can be found in clinical practice to the real needs of every patient. It is extremely encouraging that a large, worldwide group that collaborates on BE meets regularly to establish the foundations of the most basic, but highly important, definitions related to the disease, including those of exacerbation and CBI.14 There is undoubtedly a need for well-designed studies covering different phenotypes and endotypes of patients, according to their pathophysiological, clinical and microbiological characteristics, with clinically relevant endpoints beyond the mere rate of exacerbations. Such studies would elucidate more clearly which patients will need long-term antibiotic treatment or not. As always, it seems that the long-awaited precision medicine will provide the answer: “a specific treatment for every patient at a giventime”.
FundingSEPAR thought the RIBRON network.
Conflict of InterestAll authors of this editorial are also authors of the Spanish Bronchiectasis Guidelines of SEPAR published in 2018.
Please cite this article as: Martinez-Garcia MA, de la Rosa D, Cantón R, Olveira C, Máiz-Carro L, Girón R, et al. Bronquiectasias: cuando la evidencia científica publicada no resulta suficiente. Arch Bronconeumol. 2019;55:283–285.