There is no information available regarding the relationship between the respiratory symptoms or lung function and bronchial inflammation, measured by induced sputum.
ObjectivesDescription of the clinical characteristics, radiographic images and lung function of patients suffering from Primary Sjögren Syndrome (pSS), and to assess the relationship with the inflammatory airway profile.
MethodsWe analyzed clinical, radiology, lung function tests, bronchial hyperresponsiveness and inflammatory data in the induced sputum from 36 consecutive patients with pSS.
ResultsA total of 58% of patients had hoarseness and 42% had cough and dyspnea. No lung dysfunction was observed, although 46% (n=16) had a positive bronchial response. Lymphocytosis >2.6% in induced sputum was observed in 69% of all sputa. There was chronic cough in 29% of patients with lymphocytosis (n=24), whereas 73% were normal (n=11) (P=.02). The duration of cough was less for the former (p=.02). On the contrary a positive bronchial response was associated with lymphocytosis >2.6% (p=.02). Lipophages were present in 55% of pathological sputa (n=22) (index >15) versus 18% of the non-pathological ones (n=11) (p=.05).
ConclusionHoarseness, cough and dyspnea are frequent respiratory symptoms in pSS, although there is a wide variation in the relationship with bronchial responsiveness and airway inflammation. Lymphocytosis in the airways is another site of the infiltrative process in pSS, and the induced sputum is a complementary tool in the identification of active inflammatory process.
En el síndrome de Sjögren primario (SSp) no se dispone de información suficiente que analice la relación entre la clínica respiratoria o la función pulmonar y la inflamación bronquial presente, medida por esputo inducido.
ObjetivoDescripción de las características clínicas y de función pulmonar en los pacientes diagnosticados de SSp y su relación con el perfil inflamatorio de la luz bronquial.
MétodosSe analizaron síntomas respiratorios, radiología, función pulmonar, hiperrespuesta bronquial e inflamación mediante esputo inducido de 36 pacientes consecutivos diagnosticados de SSp.
ResultadosEl 58% de los pacientes presentó carraspera y el 42% tos y disnea. No hubo alteraciones destacables de la función pulmonar, pero el 46% (n=16) presentó una prueba de respuesta bronquial positiva. La linfocitosis >2.6% en esputo estaba presente en el 69% de los esputos analizados. Presentaron tos crónica el 29% de los pacientes con linfocitosis (n=24), frente al 73% de los normales (n=11) (p=0.02), con una duración de la tos inferior para el primero (p=0.02). Por el contrario, la hiperrespuesta bronquial se asoció con linfocitosis (p=0.02). El 55% de los esputos patológicos (n=22) presentaron lipófagos (índice >15) frente al 18% de los normales (n=11) (p=0.05).
ConclusiónLos síntomas respiratorios (carraspera, tos y disnea) son frecuentes en el SSp aunque su relación con la hiperrespuesta bronquial y la inflamación de la vía aérea es variable. El hallazgo de linfocitosis en la vía aérea constituye un foco infiltrativo más de la enfermedad, siendo el esputo inducido una herramienta complementaria en la valoración de la actividad inflamatoria pulmonar del SSp.
Primary Sjögren's syndrome (pSS) is a systemic disease characterized by a chronic autoimmune inflammatory process. The most relevant anatomopathologic finding that characterizes it is the presence of a focal lymphocytic infiltration of the exocrine glands, being one of the main diagnostic criteria. The inflammation leads to glandular destruction and atrophy that evolves towards mucosal dryness, mainly ocular (keratoconjunctivitis sicca), oral (xerostomy), tracheal (xerotrachea) and vaginal (vaginal xerosis).1,2 The lungs share with the exocrine glands a similar structure; therefore, their affectation can become evident during the course of the syndrome. The most frequent respiratory symptoms are chronic cough and dyspnea,3,4 which can reflect an affectation of the bronchial tree or of the pulmonary parenchyma.5–7 In the literature, the series of cases that have studied the physiopathology of the cough and dyspnea in pSS implicate an inflammatory pattern of the pulmonary interstitium and/or a concomitant affectation of the peripheral airway, mainly,3,8,9 although a poor correlation has been reported among symptoms, histopathology, lung function and radiology in pSS.9–11 On the other hand, it is unknown whether the inflammation present in the bronchial lumen, which has not been studied fully, can reflect either an early or a late stage in the natural history of the syndrome in those patients who meet the 2002 consensus diagnostic criteria12 and, therefore, if histopathology should instead be considered the gold standard pattern of the disease.2 The objectives of this study are to describe the clinical characteristics of the lung function and the inflammation present in the bronchial lumen of patients diagnosed with pSS, and to analyze whether the inflammatory profile, obtained in a non-invasive manner by means of induced sputum analysis, is associated with the presence of respiratory symptoms.
Patients and MethodsStudy PopulationThe study population was recruited from patients who were seen consecutively in an outpatient rheumatology consultation and who met the pSS consensus criteria (American College of Rheumatology consensus criteria)12 using subjective and objective data for xerostomy and keratoconjunctivitis sicca and one of the criteria for autoimmunity. These include the finding of one or more foci of inflammatory infiltrates detected in the biopsy of the minor salivary glands (Chisholm–Mason grades 3–4: at least one focal lymphocytic infiltration of ≥50 lymphocytes/4mm2) and/or the presence of Ro/La+ autoantibodies, together with the presence of symptoms of keratoconjunctivitis sicca or xerophthalmia and positivity for one of the following tests: Schirmer's, Rose bengal, sialometry, sialography, gammagraphy, ultrasound or MRI of the salivary glands. We excluded those patients who presented one of the following criteria: SS secondary to connective tissue disease, active smoker, cough-producing medication (e.g. ACE inhibitors), chemotherapy, rhinosinusitis, diagnosis of gastroesophageal reflux, chronic pneumopathies (specifically asthma, occupational or induced by radiotherapy).
The research protocol of the study was approved by the Ethics Committee of Santa Creu i Sant Pau Hospital. After having been informed and accepting voluntary participation in the study, informed consent was obtained from each patient, and we proceeded with collecting the data detailed in the protocol if the patient met the inclusion criteria.
VariablesWe identified variables for demographics (age and sex), systemic affectation symptoms (ocular, oral, otorhinolaryngeal, other affectations) and specific respiratory symptoms (dyspnea, cough and wheezing). The following variables were defined: initial symptom (first symptom appearing of the syndrome), dominant symptom (reported as being most bothersome or constant), time elapsed from the onset of the first symptom of the syndrome until the sputum sample was taken and the time elapsed from the diagnosis until the sputum sample. Radiological patterns and lung functions were studied: spirometry (FVC, FEV1), lung volumes (TLC, RV) and diffusion (DLCO and KCO), with methacholine bronchial provocation (PC20). The bronchial inflammatory pattern was obtained by means of the differential cell count of the induced sputum. Sputum was considered to have a normal profile when it presented cellularity below the 90th percentile of the reference values, and pathological if it presented higher levels.13
TechniquesLung FunctionIn accordance with the recommendations of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR),14 the following tests were carried out on different days.
For forced spirometry with bronchodilator test with the inhalation of 200μg of salbutamol, a Datospir 500 spirometer was used (Sibelmed® S.A., Barcelona).
The volumes were measured by body plethysmography using SensorMédics® equipment (Denmark) after performing between 4 and 8 correct ITGV maneuvers and later determining the residual volume by spirometry.
Carbon monoxide transfer was studied with SensorMédics® 2450 equipment (Denmark), analyzing between 2 and 4 correct maneuvers.
The nonspecific bronchial challenge test with methacholine (mg/ml) was performed with increasing doses of concentration, following the Parker method.15 Tests with inhaled accumulated concentrations that caused a fall of 20% in FEV1 (PC20) were considered positive.
Radiological TestsWe evaluated the conventional chest radiograph and computed tomography done within the three months prior to the consultation. In the event that these tests were not done, they were ordered.
Non-Invasive Test for Bronchial Inflammation (Induced Sputum)Samples were obtained after induction with 3% hypertonic saline with an ultrasound nebulizer (Omron NE U07). We selected the mucus plugs of the saliva and they were treated with dithiothreitol (DTT) (Sputolysin®, Calbiochem, Corp., San Diego, CA) at 1:10. The volume of DTT added was 4 times the weight in mg of the selected plugs, plus the same volume of phosphate buffered saline (PBS). By means of hemocytometer and trypan blue stain, we evaluated the viability of the cells in suspension, their concentration (cells/g of sputum) and the percentage of squamous cells were considered as contamination of the upper airways. After centrifuging, cellular sediment was obtained that was used to determine the percentage of lymphocytes, macrophages, neutrophils and eosinophils, using the May–Gründwald–Giemsa stain, according to the procedure described by Pizzichini et al.,16 by a trained technician who did not know the origin of the sample. The reference values used were those estimated for healthy adults.13 In addition, lipophages were determined using the oil red O stain.17
Statistical AnalysisWe used the mean values and their standard deviation to describe the variables analyzed in the sample and their comparison between the two groups of inflammation considered. For the comparison of the averages between the groups, we used the non-parametric test by Mann–Whitney for the analysis of two independent samples, and the χ2 test for the comparison of categorical variables. A P value <.05 was considered statistically significant. The data analysis was carried out with the 17.0 version of the SPSS program.
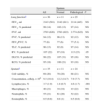
ResultsThirty-six patients were included in the study, with a mean age of 63, 10.4 (SD), 92% of whom were women. 89% presented labial biopsy with lymphocytic infiltration, 31% Ro/La autoantibodies and 50% FR/ANA autoantibodies. The mean time since the onset of the first pSS symptom was 10, 5 (SD) years and since diagnosis 6, 5 (SD) years. Table 1 shows the distribution of frequencies of the most relevant symptoms in the presentation of the syndrome, the most predominant symptoms and those most frequently combined with respiratory symptoms (clearing of the throat, dyspnea or cough). Clearing of the throat was present in 58% of the patients at some time during their evolution, cough and mild dyspnea (MRC scale=2) in 42% of the patients. The mean time with cough was 24, 40 (SD) months.
Clinical characteristics of the patients with primary Sjögren's syndrome (n=36).
| Percentage, % | Initial symptoms | Dominant symptom | Respiratory symptoms (clearing of the throat, cough, dyspnea) |
| Keratitis sicca | |||
| Xerophthalmia | 58 | 44 | 53 |
| Xerostomia | 20 | 36 | 33 |
| Clearing of the throat | 0 | 3 | – |
| Cough | 8 | 6 | – |
| Othera | 14 | 11 | 14 |
In 10 (28%) of the patients, some kind of radiological alteration of the thorax was detected with either local or diffuse affectation (interstitial or attenuation of the radiological lung density), and an associated alteration was confirmed in the volumes and/or in the diffusion in half (n=5). In 7, the sputum presented lymphocytosis higher than 2.6%.
The lung function tests were carried out and the inflammatory profile found in the bronchial lumen, both in the entire sample as well as in the two groups of patients considered, depending on whether the sputum was considered normal or pathological, are shown in Table 2. There were no relevant alterations in the lung function in the entire study sample, except for the residual volume that was higher in the group of subjects with pathological sputum.
Lung function and inflammatory profile of the bronchial lumen in patients with primary Sjögren's syndrome.
| Sputum | ||||
| All | Normal | Pathological | P | |
| Lung functiona | n=36 | n=11 | n=25 | |
| FEV1, ml | 2143 (501) | 2142 (611) | 2114 (445) | NS |
| FEV1, % predicted | 99 (14) | 102 (13) | 97 (14) | NS |
| FVC, ml | 2793 (620) | 2763 (622) | 2.774 (622) | NS |
| FVC, % predicted | 94 (12) | 96 (13) | 92 (12) | NS |
| FEV1/FVC, % | 77 (6) | 77 (6) | 77 (5.1) | NS |
| TLC, % predicted | 96 (13) | 92 (9) | 97 (14) | NS |
| RV, % predicted | 107 (22) | 97 (18) | 113 (23) | .05 |
| DLCO, % predicted | 98 (22) | 107 (31) | 95 (16) | NS |
| KCO, % predicted | 95 (18) | 100 (21) | 93 (18) | NS |
| Sputuma | n=35 | n=11 | n=24 | |
| Cell viability, % | 69 (20) | 70 (20) | 68 (21) | NS |
| Concentration, cells/g, ×106 | 9.3 (10.4) | 12.2 (14.7) | 7.9 (7.7) | NS |
| Lymphocytes, % | 3.7 (1.9) | 1.8 (0.7) | 4.6 (1.7) | .001 |
| Macrophages, % | 40 (21) | 34 (18) | 43 (22) | NS |
| Neutrophils, % | 55 (21) | 61 (20) | 52 (22) | NS |
| Eosinophils, % | 0.5 (0.8) | 0.8 (1) | 0.5 (0.8) | NS |
DLCO: alveolar diffusion of carbon monoxide; FEV1: forced expiratory volume in one second, in milliliters or in percent predicted; FEV1/FVC: ratio of the forced expiratory volume in one second of the forced vital capacity; FVC: forced vital capacity in milliliters or in percentage of the predicted value; KCO: diffusion corrected with alveolar volume; NS: not significant for the statistical level contemplated; TLC: total lung capacity; RV: residual volume.
Of all the sputum analyzed (n=35), 69% (n=24) were considered pathological, while the remaining 31% (n=11) were normal. The patients who presented pathological inflammatory profiles did so exclusively at the expenses of the lymphocytes (>2.6%). There were no statistical differences between the two groups regarding time elapsed since the appearance of the first symptom: 11, 6 (SD) in those who presented pathological sputum compared with 9, 3 (SD) years in those with normal sputum. The same was true for the time elapsed since diagnosis: 7, 5 (SD) in those who presented pathological sputum compared with 6, 4 (SD) years in patients with normal sputum.
Table 3 shows the characteristics of the cough and the bronchial response with bronchodilation or methacholine, according to the inflammatory profile found in the bronchial lumen.
Characteristics of cough and bronchial response of patients with pSS according to the inflammatory profile found in the bronchial lumen.
| All | Sputum | |||
| Normal | Pathological | P | ||
| Cough | n=35 | n=11 | n=24 | |
| No. and percentage, n (%) | 15 (43) | 8 (73) | 7 (29) | .02 |
| Duration of cough, monthsa (X, SD) | 24 (40) | 51 (56) | 13 (23) | .02 |
| Bronchial hyperresponse | ||||
| PC20, n (%) | 12 (33) | 2 (18) | 10 (42) | NS |
| Bronchodilator test, n (%) | 6 (17) | 1 (9) | 5 (21) | NS |
| PC20 or BD test | 16 (46) | 2 (18) | 14 (58) | .02 |
BD test: bronchodilator test after inhaling 200μg of salbutamol; PC20: accumulated concentration of inhaled methacholine that caused a fall of 20% in FEV1 (forced expiratory volume in one second).
In all the sputum samples except three, the lipophage count was analyzed (macrophages with presence of lipid inclusions). 42% (n=14) presented a pathological index higher than 15 (range of count 0 to 400), distributed in 55% (n=12) of the pathological sputum (n=22) compared with 18% (n=2) of the normal sputum (n=11), P=.05.
DiscussionThe majority of patients with pSS present respiratory symptoms (clearing of the throat, cough and dyspnea) at some time during the evolution of their disease, along with other concomitant symptoms of extraglandular affectation (ocular, oral, etc.). These are more predominant than the respiratory symptoms in the evolutionary course of the disease, especially at the beginning. The prevalence and mean time from the onset of the systemic and respiratory symptoms described in this series coincide with those reported in the literature, with dyspnea and cough being the most relevant among the respiratory symptoms.1,3,5,18 In our series, chronic cough was the respiratory symptom that presented a more prolonged duration over time and was combined with other symptoms of ocular or oral dryness. It was not the initial or predominant symptom among those detailed, although its verification should lead to the suspicion of pSS diagnosis in the management of chronic cough.19 Cough has been postulated as a main symptom of tracheal affectation above other respiratory symptoms in the glandular affectation of pSS. It may be accompanied by dyspnea in the presence of radiological affectation or alteration of the lung function in the extraglandular form, resulting in parenchymatous and bronchiolar affectation.9 Mention should be made that, in the patients we analyzed, in those who coughed more and in those whose cough lasted longer, sputum was normal (Table 3).
On the other hand, in the entire series studied the radiological and lung function affectation was mild in some patients, although its detection implicates a close follow-up of the symptoms. These findings agree with the descriptions of the literature and with the limited radiological and lung function associations over time reported for pSS.8,10,11,20 It should also be remarked that in the majority of this subgroup of patients with radiological or pulmonary affectation, the sputum presented lymphocytosis.
33% of the cases presented positive bronchial challenge with methacholine, and 17% presented positive bronchodilator test. Together, these percentages should be considered as high overall, and possibly reflect a tracheobronchial immunological affectation with hyperresponse, as has already been suggested by the series of La Corte et al.21 and Gudbjörnsson et al.22 Although when analyzed separately these hyperresponse tests do not demonstrate a different profile in the sputum, when the presence of one or the other is confirmed and they are grouped for analysis, the predominant profile in the sputum is lymphocytosis (Table 3). Therefore, this hyperresponse would be that of a non-specific response of the airway dysfunction with a difficult physiopathological translation, but it would implicate the affectation of the glands of the tracheobronchial mucosa,23 the submucosa or the alveolar bed.3,7
Among all the results of our study, what is especially relevant is the inflammatory profile found in the non-invasive samples collected from the induced sputum, which were two-thirds pathological. The presence of lymphocytosis in the majority of the subjects of our series is in accordance with the alveolar inflammatory cell affectation predominant at the parenchymatous and bronchioloalveolar level published for this syndrome.3–5,7 Thus, we can affirm that its presence in the bronchial lumen indicates a very defined immunological affectation of the airway. This anatomopathologic alteration, in our opinion, guarantees the presentation of a persistent affectation of the tracheobronchial glandular physiology and of the airway. It also affirms that the bronchial localization is yet another of the anatomical foci to be considered in the diagnostic approach of pSS, given its good accessibility with induced sputum and its probable correlation with the lymphocytosis present in the bronchoalveolar lavage.3,6 It may be a valid diagnostic alternative to waiting to confirm pSS with a compatible biopsy of the labial mucosa, or rather, the verification of a more extensive or severe syndrome. Moreover, its presence in the airway is directed along the same critical line as the definition of SS postulated by Ramos-Casals et al.2 Given contemplation, this could contribute to changing the denomination of Sjögren's syndrome to that of Sjögren's systemic disease if persistent lymphocytosis is demonstrated at the bronchial level in patients with dry syndrome.2 For this reason, we consider that lymphocytosis is a relevant finding that is important to verify in patients with pSS and that it could possibly contribute to improving the classification of the immunological severity of the disease. Thus, the analysis of induced sputum can be considered a useful tool with a significant role in the evaluation of pSS patients.
On the other hand, it should also be noted that the majority of the sputa with lymphocytosis analyzed were accompanied by lipophages. This fact can be explained by the physiological affectation of the gastrointestinal mucosa by the disease itself, which would favor the microaspiration of gastric content to the bronchial Tree,6 or rather it simply reflects a greater degradation of the local glandular cell destruction. In our study, the presence of gastroesophageal reflux could not be confirmed by esophageal pH, although this fact has already been published in pSS.6
In this study, the following limitations can be considered. First of all, although it could be argued that there was a systematic appearance of lymphocytosis in the bronchial sample after nebulization depending on the induction time, this would equally affect all the subjects who underwent the same induction protocol and should have been found in all the subjects and not in just some. Nevertheless, although very improbable, it is not possible to completely rule out a partial recruitment of lymphocytes depending on the time and as an effect of the induction.24
In our study, we could not control the effect of systemic or inhaled medication that the patients were receiving, as it was very heterogeneous, therefore its influence on the interpretation of the duration of cough, lymphocytosis in sputum and methacholine was not evaluated.
In conclusion, respiratory symptoms (clearing of the throat, cough and dyspnea) are frequent in pSS, although their relationship with bronchial hyperresponsiveness and airway inflammation is variable. The lymphocytosis of the airway is the most constant pathological inflammatory finding, which probably defines, more than a syndrome, Sjögren's systemic disease respiratory affectation. Induced sputum is a useful tool in the evaluation of the bronchial affectation of pSS.
Conflict of InterestThe authors declare that they have no conflict of interest.
We would like to thank Dr. José Belda and Ms. Aparicia Ramos for their support in the completion of this study.
Please cite this article as: Bellido-Casado J, et al. Inflamación bronquial, clínica respiratoria y función pulmonar en el síndrome de Sjögren primario. Arch Bronconeumol. 2011;47:330–4.