The incidence and severity of lung toxicity reported in clinical trials with bortezomib, a synthetic anticancer drug, was low. However, since marketing, severe cases have come to light.1,2
We report the case of a 65-year-old female patient with a diagnosis of multiple myeloma (MM) presenting with acute dyspnea, fever and pulmonary infiltrates after receiving combined PAD chemotherapy (bortezomib, adriamycin and dexamethasone).
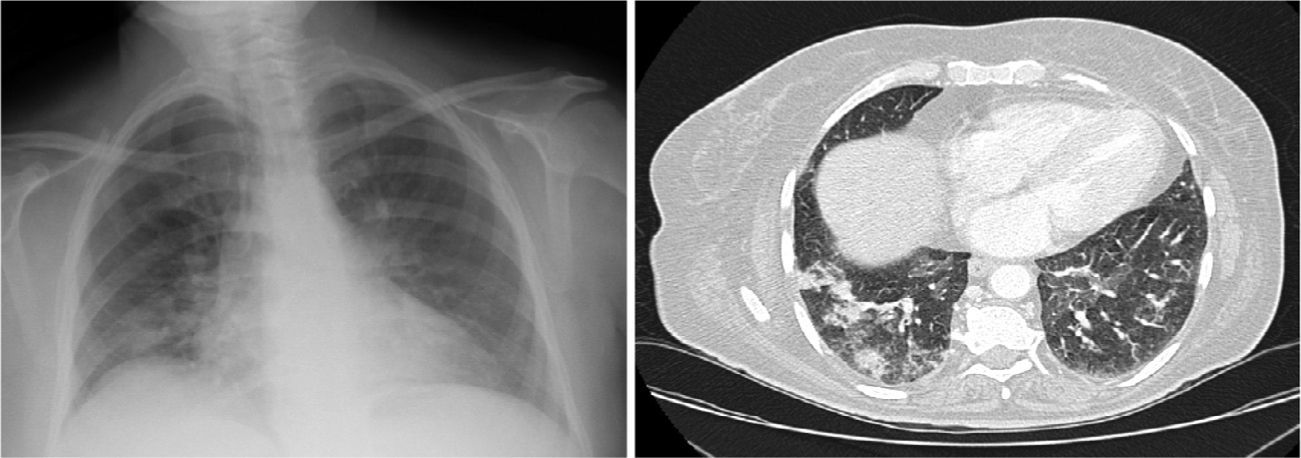
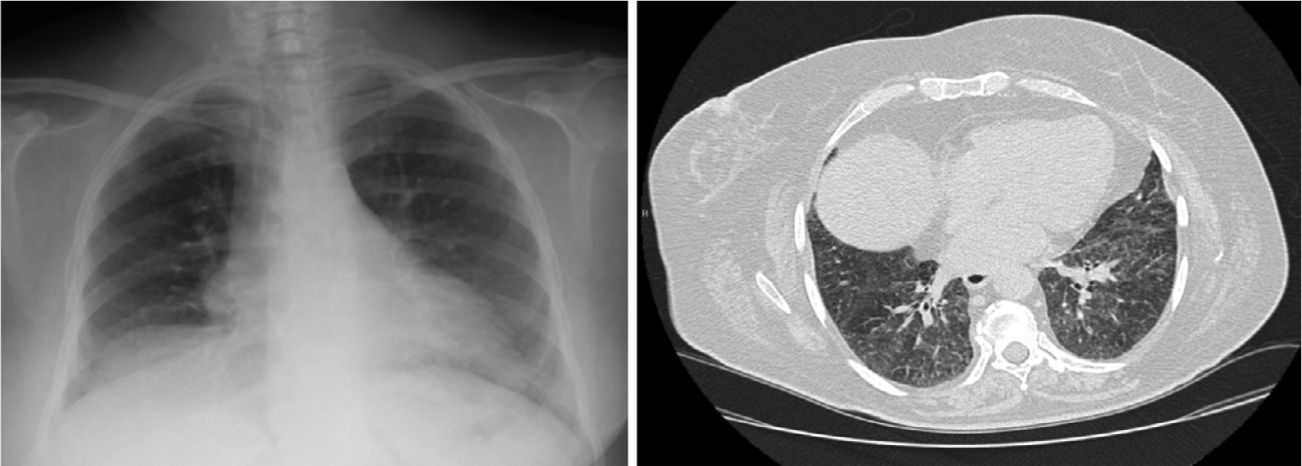
Clinical CaseA 65-year-old woman with history of hypertension, no toxic habits, diagnosed with stage IIIA IgG lambda-type MM. After receiving her third cycle of PAD, she developed a clinical picture of dyspnea, dry cough and fever. Chest X-ray and computed tomography (CT) revealed bilateral pulmonary infiltrates (Fig. 1A and B). Treatment began with piperacillin/tazobactam, amikacin and linezolid. Blood gases showed type 1 respiratory failure with PaO2 of 49.2mmHg. Biochemistry and hematology results were normal. Results of microbiological testing, including sputum cultures, blood cultures, specific antigens for atypical pneumonia in urine and serum, were negative. Fiberoptic bronchoscopy revealed a normal bronchial tree with sparse whitish secretions. Results of bronchial aspirate and bronchoalveolar lavage (BAL) cultures were negative. Transbronchial biopsy revealed fragments of pulmonary parenchyma with foci of alveolar desquamation, mild mixed inflammatory infiltrate and bleeding with absence of malignant infiltration, granulomas or fibrosis. No microorganisms were identified.
Drug-induced lung toxicity was suspected, and treatment began with prednisone 1mg/kg/day in a tapering regimen, until discontinuation 1 month later.
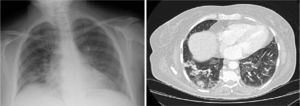
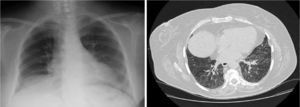
Seven months later, high resolution CT showed improvement of pulmonary infiltrates, persistent patchy ground-glass images with cobblestone pattern and diffuse, irregular thickening of the peribronchovascular, centrolobular and peripheral septal interstitium (Fig. 2).
A new chemotherapy regimen with lenalidomide+dexamethasone was introduced, with good tolerance and hematological response after 5 cycles.
Spirometry performed 4 months after discharge showed a restrictive pattern (FVC: 58%).
DiscussionBortezomib is an anticancer drug initially marketed for second-line, single-agent treatment in progressive MM. It is currently used in combined chemotherapy in association with melfalan, cyclophosphamide and doxorubicin.
The incidence of severe adverse effects in clinical trials was lower than 5%, and severe pulmonary complications were not described before the publication of a series of 4 cases.1
The pathophysiological mechanism of bortezomib lung injury is not well established. Several theories have been put forward, for example: bortezomib withdrawal might reactivate NF-κB1; it is a direct consequence of tumor lysis syndrome; or it is form of pauci-immune capillaritis.3
Various radiological patterns, none of which are specific, have been described, including ground glass opacification, consolidations on air bronchogram, nodules and pleural effusion.
No characteristic histopathological pattern has been identified on either transbronchial lung biopsy or in BAL cytology.
Diagnosis is based on a suggestive clinical picture coinciding with a feasible time frame, after exclusion of other possible triggers, such as infectious disease or pulmonary involvement of the underlying malignant process.
The use of bortezomib has been associated with the development of an obstructive spirometric pattern,4 but in our patient the opposite was observed.
The therapeutic approach is mainly three-fold: immediate drug withdrawal, supportive measures and the administration of glucocorticoids. Nevertheless, widely ranging responses have been reported.1,2,5
Rapid identification of possible “sentinel episodes” of lung injury is essential, so that the clinician can be alerted to the possibility of a potentially fatal disease and the appropriate measures can be implemented.
Please cite this article as: Santalla Martínez M, Blanco Cid N, Dacal Quintas R. Toxicidad pulmonar inducida por bortezomib. Arch Bronconeumol. 2014;50:564–565.