Bronchiolitis obliterans (BO) is the most common expression of chronic allograft dysfunction in lung transplantation. Moreover, BO represents the major cause of death in the long-term after this procedure. On the other hand, mesenchymal stem cells have been tested in animal models of BO aiming to interfere in its development. The aim of this experimental study is to explore the role of bone-marrow derived stem cells (BMSCs) as a preventive intervention of BO occurrence.
Materials and methodsThis an experimental randomized study. A bronchiolitis obliterans animal model in rats was reproduced: heterotopical tracheal transplant model in lung parenchyma. Five of these animals were used as control group. After setting up the model, individuals were divided in 3 groups of treatment (n=15), in which BMSCs were administered in 3 different time points after the tracheal transplant (tracheal transplantation and BMSCs administration occurred the same day, group G0; after 7 days, group G7; after 14 days, group G14. In addition, within each group, BMSCs were administered through 3 different routes: endotracheally, endovascular and topically in the lung parenchyma). Animals were sacrificed at 21 days. Histology, fluorescence in situ hybridization and immunohistochemistry techniques were performed for identifying stem cells.
ResultsCompared to control group, animals receiving BMSCs showed large neovessels in a loose fibrous matrix. Group G7 showed less fibrosis (p<0.033) and edema (p<0.028). Moreover, G7 animals receiving stem cells endotracheally showed no fibrosis (p<0.008). Alveolar-like patches of tissue were observed among all groups (53.4%, 46.7% and 40% in G0, G7 and G14 respectively), consisting of cells expressing both stem and alveolar cells biomarkers.
ConclusionBMSCs modify the course of bronchiolitis obliterans and differentiate into alveolar cells. Endotracheal administration of BMSCs 7 days after the heterotopical tracheal transplant might be considered an effective way to prevent BO in this animal model.
La bronquiolitis obliterante (OB) es la forma más frecuente de disfunción crónica del injerto en el trasplante pulmonar. Asimismo, representa la principal causa de mortalidad a largo plazo tras este procedimiento. Por otro lado, las células madre mesenquimales se han utilizado en diferentes modelos animales de BO, con el propósito de interferir en el desarrollo de dicha disfunción. El objetivo de este estudio experimental es explorar el papel del trasplante de células madre mesenquimales derivadas de la médula ósea (BMSC, por sus siglas en inglés) como tratamiento preventivo de la BO.
Material y métodosSe trata de un estudio experimental y aleatorizado en el que se empleó un modelo de BO en ratas basado en el trasplante traqueal heterotópico en parénquima pulmonar. Los animales se dividieron en los siguientes grupos: grupo control (n=5) y 3 grupos de tratamiento (n=15) en los que las células madre mesenquimales derivadas de médula ósea se administraron a distintos tiempos tras el trasplante traqueal heterotópico (el mismo día [grupo G0]; a 7 días [grupo G7], y a 14 días [grupo G14]). Además, dentro de cada grupo, las células se trasplantaron mediante 3 vías diferentes: endotraqueal, endovascular y tópicamente en el parénquima pulmonar. Todos los animales se sacrificaron a los 21 días tras el trasplante traqueal. Las células madre se identificaron mediante técnicas histológicas utilizando hibridación fluorescente in situ (FISH) e inmunohistoquímica.
ResultadosComparado con el grupo control, los animales que recibieron las BMSC mostraron neovasos de gran tamaño en una matriz muy laxa de tejido fibroso. En el grupo G7 se observó menor grado de fibrosis (p<0,033) y edema (p<0,028). Además, ninguno de los animales del grupo G7 que recibieron las células madre por vía endotraqueal desarrolló algún grado de fibrosis (p<0,008). En todos los grupos se observaron parches de tejido con características histológicas similares al tejido alveolar (53,4, 46,7% y 40% in G0, G7 y G14, respectivamente) con expresión de marcadores tanto alveolares como de células madre.
ConclusionesLas células madre mesenquimales derivadas de la médula ósea modifican el curso histopatológico de la BO y son capaces de diferenciarse a células de tipo alveolar. La administración endotraqueal de estas células a los 7 días del trasplante traqueal heterotópico podría considerarse una vía efectiva para prevenir el desarrollo de BO en este modelo animal.
Lung transplantation is an established lifesaving therapy for an increasing number of patients suffering from irreversible end-stage pulmonary diseases. Although early outcomes (hospital mortality and one-year survival rates) have dramatically improved over recent years, long-term survival rates are still far from those achieved with other solid organs. In the last decade, the 5-year survival rate has remained 50%,1 which contrasts with survival rates above 70% for heart, kidney, liver or pancreas recipients.2
Chronic lung allograft dysfunction (CLAD) as a whole, and bronchiolitis obliterans syndrome (BOS) as a specific phenotype, is the main cause of death after the first year post-lung transplant1 and represents the Achilles’ heel of this therapeutic option. Half of our patients will develop BOS by 5 years and more than 70% by 10 years after transplantation.1 Its pathological expression is termed bronchiolitis obliterans (BO) and, essentially, consists of a fibrotic process affecting the small airway. In the early phases a lymphocytic infiltrate is observed in the submucosa and epithelium, which is called lymphocytic bronchiolitis.3 Later, necrotic changes and ulceration occur in the epithelium and trigger a chronic inflammatory reaction, which results in fibroblastic migration into the airway lumen. The subsequent fibroproliferative process leads to small airway partial or total obstruction.4 The etiology of this process is still unknown. Although likely multifactorial, there is increasing evidence that innate and acquired immunological factors play a crucial role in its course.5,6
Stem cells have shown a consistent ability to modulate the inflammatory response and enhance the healing process of many disorders in different target tissues.7–9 Besides, the lack of Major Histocompatibility Complex (MHC) class I and II expressions on these cells prevents immunological rejection. Furthermore, it has been described that mesenchymal stem cells applied both locally and systemically may slow the development of bronchiolitis obliterans in other animal models because of this immunomodulatory ability.10
In this paper, we aim to assess the impact of allogeneic adult bone marrow-derived mesenchymal stem cells (BMSC) in an experimental rat model of BO using different routes of administration.
Materials and methodsThis is an experimental randomized study. The experimentation committee of Hospital Universitario Puerta de Hierro-Majadahonda, Spain, approved all phases of the project.
Animal model of BOWe used heterotopic tracheal transplantation in rats as a model of BO, in accordance with our previous publication.11 Briefly, a 2–3 cartilage ring segment of trachea (allograft) from a female Lewis rat donor was grafted into the recipient left lung parenchyma, a female Wistar rat. All rats were euthanized on day 21 and allografts processed for histological analysis.
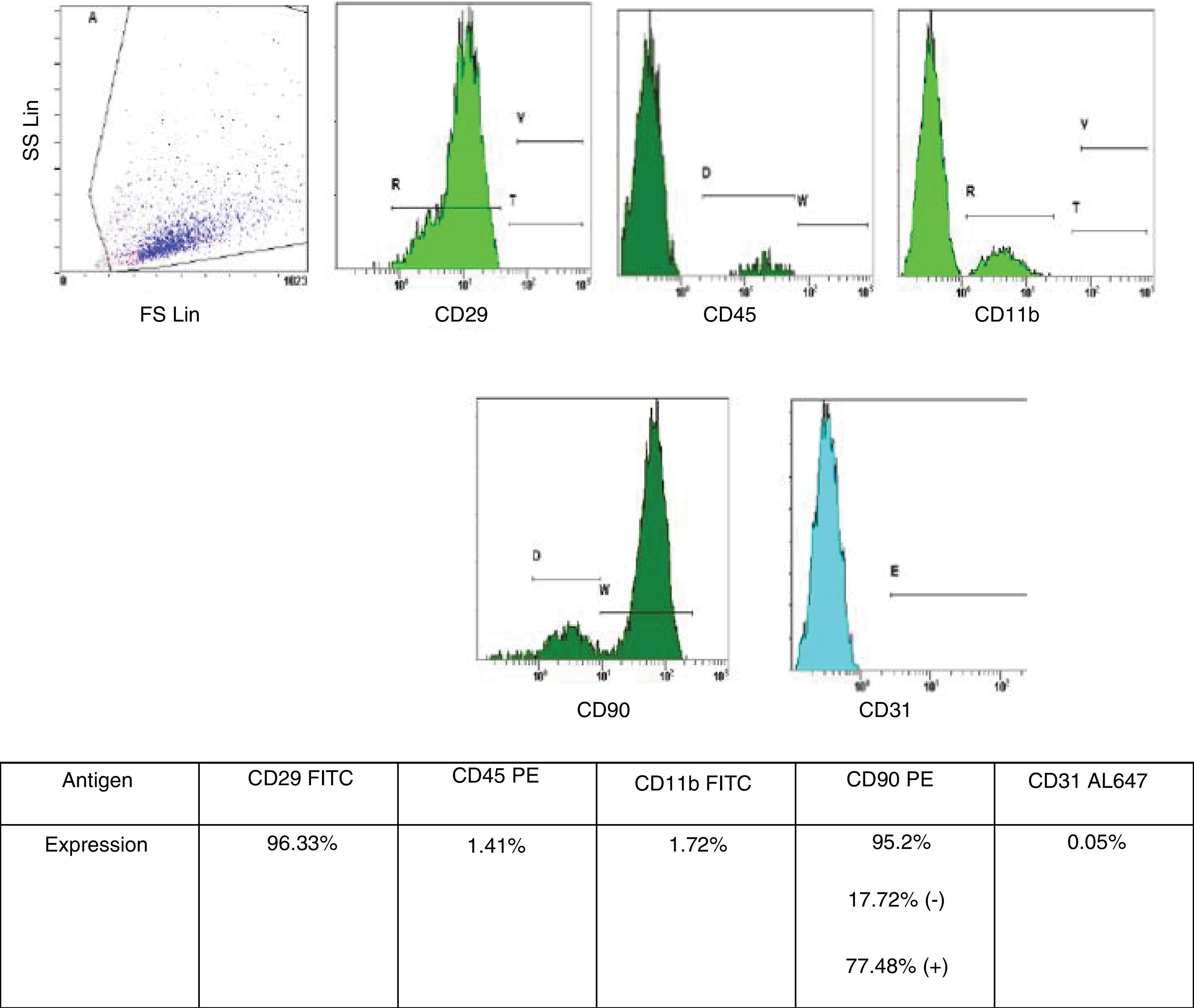
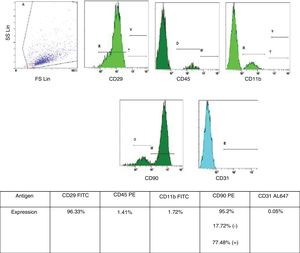
Bone marrow-derived mesenchymal stem cell (BMSC) isolation and characterizationBMSC isolation from male rats and phenotypic characterization were performed as described in previous publications from the Neuroscience Research Unit at our Hospital.12 BMSC were retrieved from tibia and femur of male rats by washing both epiphysis and diaphysis with alfa-minimun essential medium (alfa-MEM). Bone marrow was collected and dissociated by mechanical methods. The obtained homogeneous sample was then filtered and incubated at 37°C in 5% CO2 for 5 weeks. For characterization of the BMSC phenotype, we used flow cytometry analysis (Cytometer Cytomics FC500 MPL – Beckman Coulter, Inc., Brea, CA, USA, and CFC 500MXP software, version 2.2 – Beckman Coulter Inc., Miami, FL, USA). The following cell-surface markers were characterized CD11b, CD29, CD31, CD45 and CD90, with a suitable fluorochrome-conjugated isotype control (all antibodies were from AbD Serotec – Oxford, United Kingdom, all specific to rats). Only cells under 5 weeks in culture and with phenotypic characteristics for MSC published by International Society of Cellular Therapy (i.e., expression of CD29 and CD90 [95% positive] and absence of expression of CD11b, CD45 or CD31 [<5% positive]) were used (Fig. 1).13
Control and experimental groups, and BMSC applicationOnce the tracheal segments were grafted in the recipients (n=50), they were divided into a Control Group (CG), n=5, and 3 experimental groups (15 rats each) depending on the day on which the BMSC were transplanted: group 0 recipients (G0) received BMSC the same day of tracheal segment grafting; group 7 (G7) received BMSC on day 7; and group 14 (G14) received BMSC on day 14. Each experimental group was divided into subgroups (5 animals each) depending on the route used for BMSC application: endotracheal (ET), intravenous (IV) or lung injection (LI). In order to preferentially direct the BMSCs toward the left lung, which contained the tracheal graft, rats were placed in left lateral decubitus for ET administration. BMSCs were then injected through the endotracheal tube. For IV injection we used a jugular access with neck dissection as it has previously been showed that revascularization of the tracheal graft is derived from the pulmonary circulation.11,14 Finally, for LI transplantation, we reopened the thoracotomy and injected BMSC in the tissue around the allograft.
Different BMSC doses were administered depending on the selected route, ensuring the maximal concentration of cells without causing vascular or airway clots. Through the ET route, 7×106 cells suspended in 500μl of autologous plasma were transplanted (concentration of 14×106/ml); LI route, 15×106 cells suspended in 250μl of autologous plasma (concentration 60×106/ml); and EV route, 1.5×106 cells suspended in 300μl of autologous plasma (concentration of 5×106/ml).
All allografts were retrieved after 21 days, as described before.
Histology, BMSC detection and immunohistochemical stainingAfter 21 days of tracheal segment grafting, all individuals were euthanized by transcardiac injection of potassium (1ml) after inhaled anesthesia with 8% sevoflurane. The tracheal allograft and a portion of surrounding lung parenchyma were recovered and processed for paraffin sectioning (2.5–4μm). At least 2 sections were used for Hematoxylin–Eosin (H–E) and trichromic staining and another 2 for BMSC detection.
Fluorescent in situ hybridization (FISH) technique was performed for BMSC detection12 by using specific biotinylated DNA that links the murine Sry gene sequence in the Y-chromosomes (sex determining region). The only cells showing Y-chromosomes are BMSC obtained from male donor rats. The biotinylated DNA probe was visualized by immunofluorescence using a primary antibody-IgG monoclonal mouse anti-biotin (1:100, Jackson), and a secondary antibody-rhodamine IgG-conjugated anti-mouse (1:200, Jackson). Both negative and positive controls were performed.
Immunohistochemical (IHQ) staining was done for both proliferative cell and pneumocyte II biomarkers (Ki-67 and pulmonary surfactant protein B, respectively). Ki-67 proliferation markers were determined in the same manner as previously reported.12
IHQ for protein B detection was performed in specific samples, as described in the results. These selected samples were incubated overnight with biotin-conjugated anti-mouse immunoglobulin Ig(G) anti-protein B (dilution 1:500, ab40876 Abcam, Cambridge, UK). After washing, they were incubated with a secondary anti-mouse biotin-conjugated Ig(G) antibody (1:200, Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA). All sections were washed with PBS, incubated with avidin–biotin–horseradish peroxidase complex (Vector Inc.), and 3,3′-Diaminobenzidine was used as chromogen. Negative and positive controls were performed.
VariablesDifferent histological variables were studied, such as fibrosis (grade 0 – no fibrosis, grade 1 – mild fibrosis, grade 2 – severe fibrosis), inflammation (grade 0 – nil; grade 1 – moderate, inflammatory cell local accumulations; grade 2 – severe, diffuse inflammatory infiltrates), neovascularization (grade 0 – nil or slight presence of visible vessels and endothelium; grade 1 – moderate presence; grade 2 – more significant presence), and edema (grade 0 – nil or slight; grade 1 – patchy distribution; grade 2 – diffuse distribution). Finally, we looked for the presence of BMSC in the samples.
Statistical analysisThis is a prospective, randomized, experimental study. The pathologist was blinded regarding the application or not of BMSC in the analyzed samples.
Qualitative variables were analyzed using corrected χ2 and Fisher tests. Data analysis was carried out using SPSS software v14.0 (IBM SPSS Statistics, Armonk, NY, USA). A p value of <0.05 was considered statistically significant.
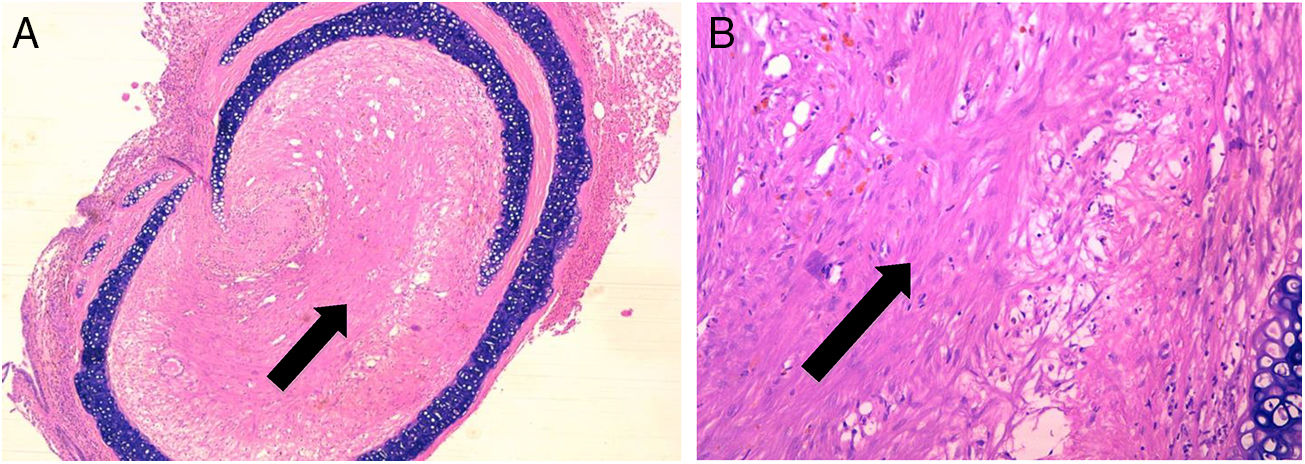
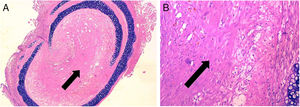
ResultsControl group is representative of the typical BO lesionAs stated in Material and Methods, we used a heterotopic rat transplantation animal model for reproducing the BO lesion. After 21 days, allografts were retrieved and analyzed (H–E staining). All 5 animals in the control group demonstrated absence of epithelium and dense collagen tissue with no inflammatory infiltrate occupying the lumen of the allograft (Fig. 2). These findings are consistent with previously published histological patterns using this model,11,14 such as loss of epithelium and base membrane, and fibrous obliteration of the lumen.
BO typical lesion observed in control group (CG) (25×, H–E). The tissue located in the lumen of the allograft is mainly formed by scar tissue (collagen and fibroblastic cells) (black arrows). (A) Overview of a section of the tracheal segment. (B) Detail of the scar tissue (collagen and fibroblastic cells).
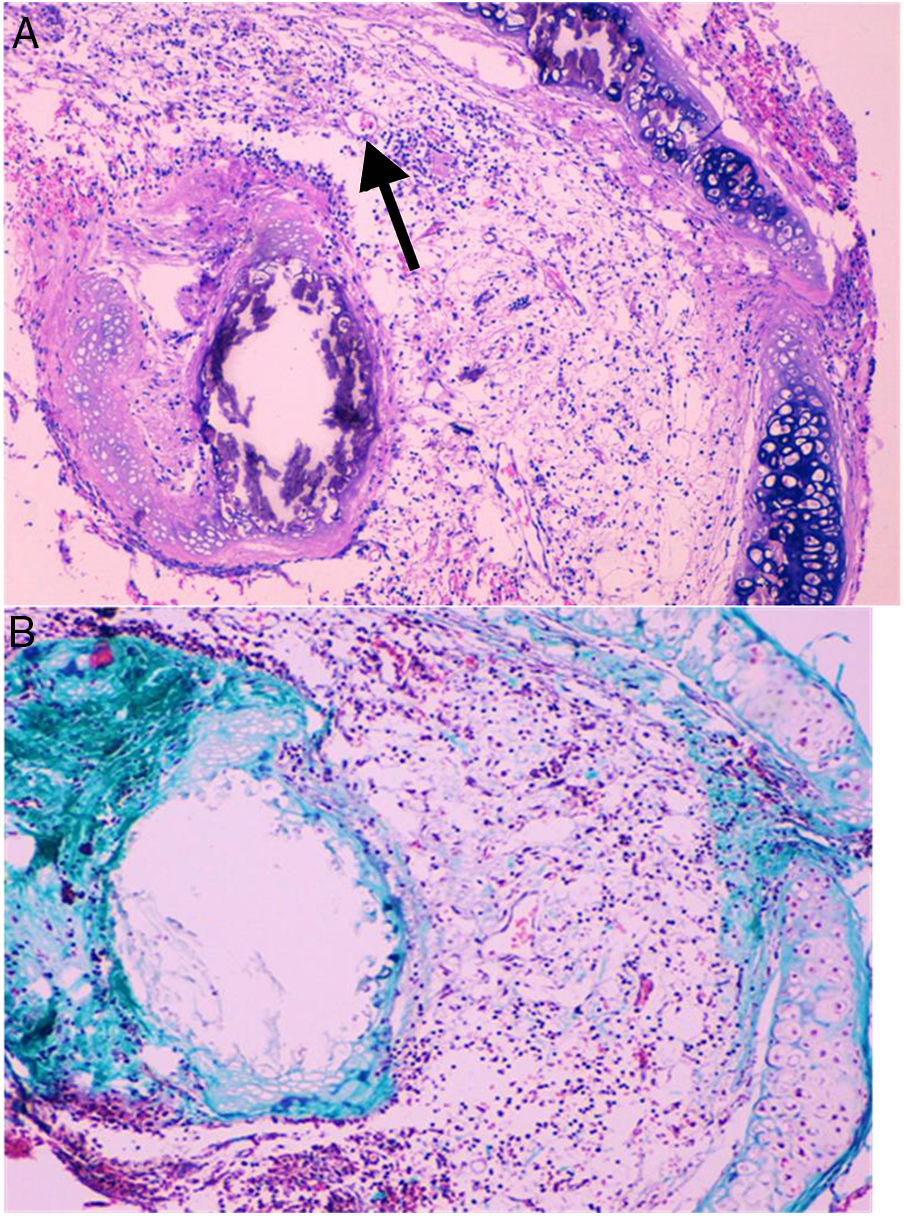
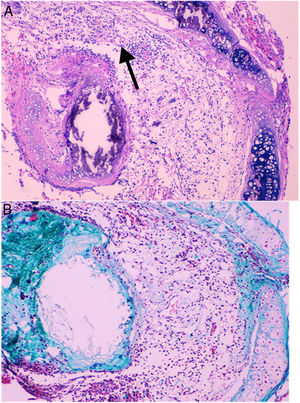
Histological qualitative analysis showed numerous large neovessels in a very loose fibrous tissue matrix with no inflammatory infiltrate (Fig. 3). Trichromic staining showed collagen deposition in both cartilaginous and membranous walls of the allograft, and a loose collagenous net inside the lumen. These findings were found in all groups.
(A) Histological findings in groups than received BMSC. These findings consist in a large number of neovessels in a very loose collagen bed (25×, H–E). The arrow is pointing to a one of the neovessels. (B) Next section of the same histologic sample showing the loose collagen tissue (green color) with trichrome staining (25×).
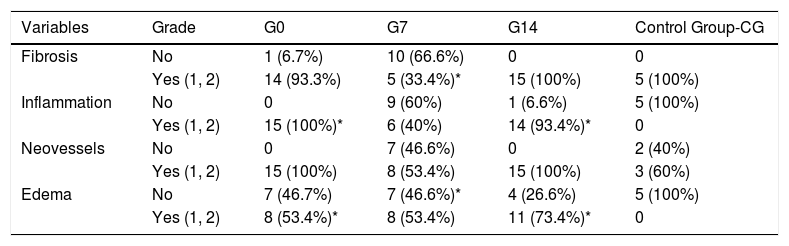
In Table 1, we present the most remarkable aspects of the histological analysis. In comparison to CG, in which no inflammation at all was found, G0 and G14 animals showed significantly more inflammatory response (p<0.001 and p=0.011, respectively), regardless the route of BMSC administration. With regard to the presence of edema, we observed that G0 and G14 rats had significantly more than CG (p=0.023 and p=0.048, respectively), also regardless the route of BMSC application.
Comparison between BMSC groups and the control group.
| Variables | Grade | G0 | G7 | G14 | Control Group-CG |
|---|---|---|---|---|---|
| Fibrosis | No | 1 (6.7%) | 10 (66.6%) | 0 | 0 |
| Yes (1, 2) | 14 (93.3%) | 5 (33.4%)* | 15 (100%) | 5 (100%) | |
| Inflammation | No | 0 | 9 (60%) | 1 (6.6%) | 5 (100%) |
| Yes (1, 2) | 15 (100%)* | 6 (40%) | 14 (93.4%)* | 0 | |
| Neovessels | No | 0 | 7 (46.6%) | 0 | 2 (40%) |
| Yes (1, 2) | 15 (100%) | 8 (53.4%) | 15 (100%) | 3 (60%) | |
| Edema | No | 7 (46.7%) | 7 (46.6%)* | 4 (26.6%) | 5 (100%) |
| Yes (1, 2) | 8 (53.4%)* | 8 (53.4%) | 11 (73.4%)* | 0 |
G0: animals receiving BMSC the same day of tracheal grafting.
G7: animals receiving BMSC 7 days after tracheal grafting.
G14: animals receiving BMSC 14 days after tracheal grafting.
None of the animals across all 3 experimental groups showed restitution of the epithelium.
Fibroproliferative responseA reparative effect that leads to fibrocollagenous tissue formation is expected within the allograft lumen in this specific animal model of BO.11,14 This sort of fibroblastic response is also initiated whenever the lung tissue experiences an injury after transplantation.15 Therefore, with the purpose of describing the different fibroproliferative responses between experimental groups G0, G7 and G14 and CG, histological qualitative analysis was performed in all samples.
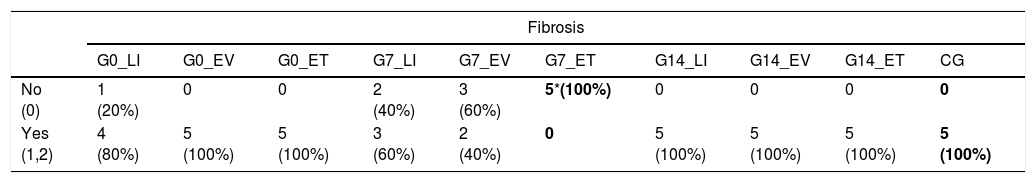
Either grade 1 or 2 fibrosis was found in most of the animals in G0 and G14. On the other hand, significantly less fibrosis was detected in G7 in comparison to CG (p=0.033). A more detailed analysis considering the routes of administration of BMSC showed, essentially, that those animals who received BMSC through endotracheal route (G7_ET) did not develop any grade of fibrosis whatsoever (p=0.008) (Table 2 and Fig. 2).
Fibroproliferative response. Comparison between BMSC transplantation routes and CG.
| Fibrosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0_LI | G0_EV | G0_ET | G7_LI | G7_EV | G7_ET | G14_LI | G14_EV | G14_ET | CG | |
| No (0) | 1 (20%) | 0 | 0 | 2 (40%) | 3 (60%) | 5*(100%) | 0 | 0 | 0 | 0 |
| Yes (1,2) | 4 (80%) | 5 (100%) | 5 (100%) | 3 (60%) | 2 (40%) | 0 | 5 (100%) | 5 (100%) | 5 (100%) | 5 (100%) |
CG: control group.
G0_LP: animals receiving lung injected BMSC the same day of tracheal grafting.
G0_EV: animals receiving intravenous BMSC the same day of tracheal grafting.
G0_ET: animals receiving endotracheal BMSC the same day of tracheal grafting.
G7_LP: animals receiving lung injected BMSC 7 days after tracheal grafting.
G7_EV: animals receiving intravenous BMSC 7 days after tracheal grafting.
G7_ET: animals receiving endotracheal BMSC 7 days after tracheal grafting.
G14_LP: animals receiving lung injected BMSC 14 days after tracheal grafting.
G14_EV: animals receiving intravenous BMSC 14 days after tracheal grafting.
G14_ET: animals receiving endotracheal BMSC 14 days after tracheal grafting.
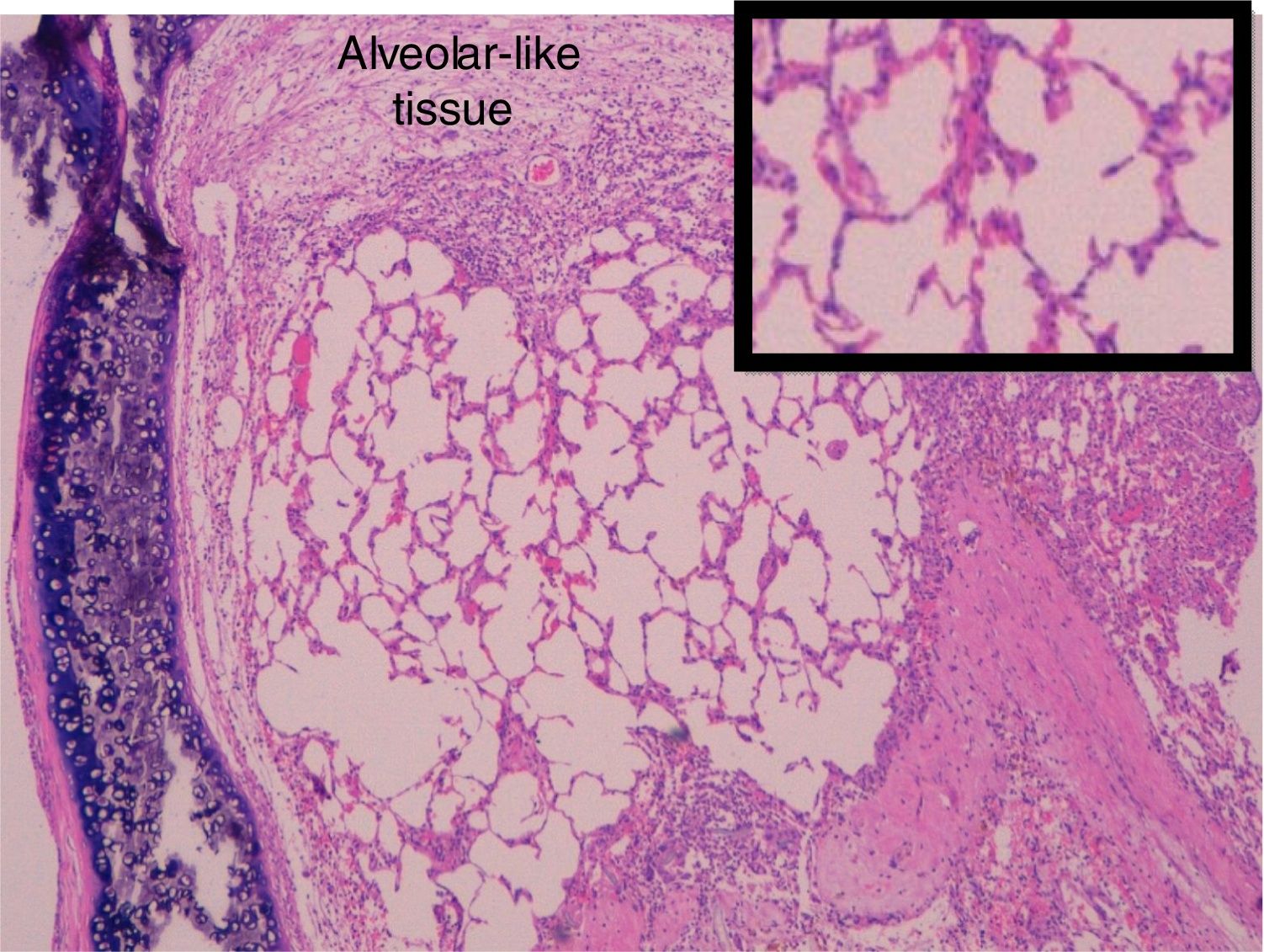
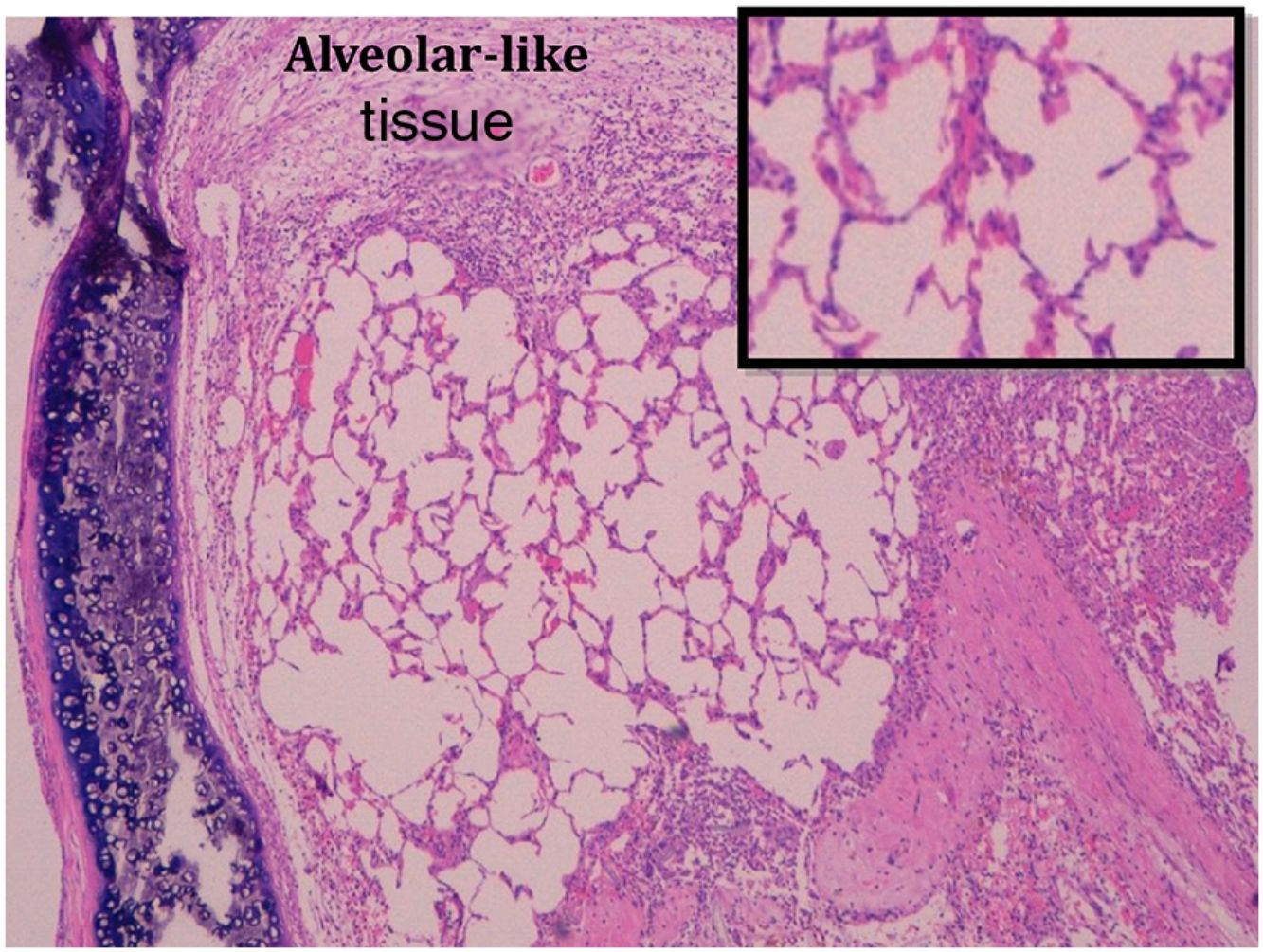
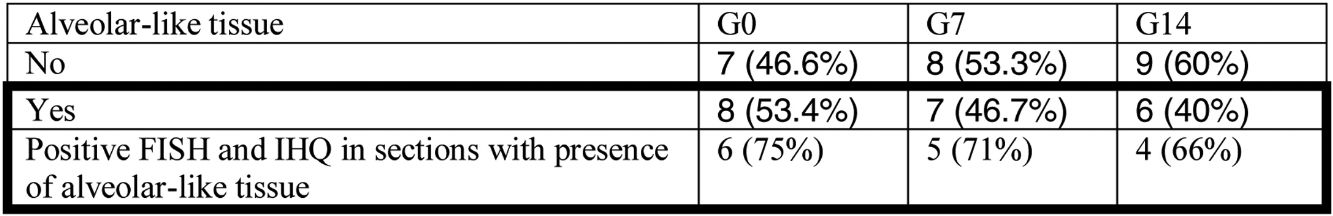
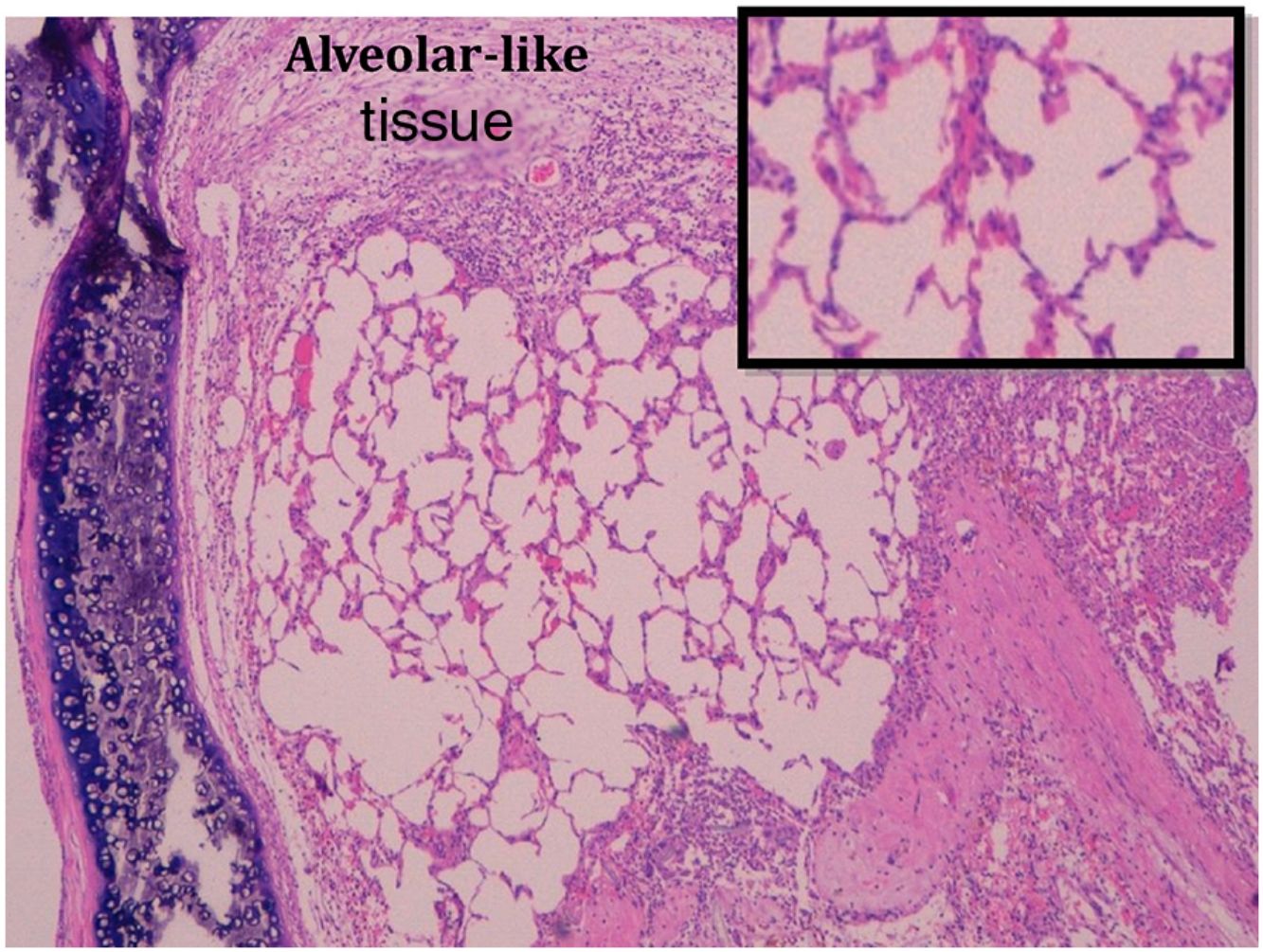
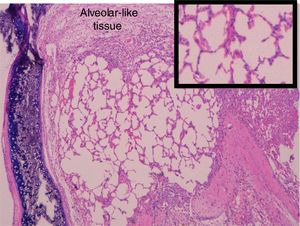
Administration of BMSC has previously demonstrated the ability to enhance lung regeneration.16 In the present study, patches of epithelial tissue with histological architecture similar to alveoli were observed within the allograft lumen (Fig. 4). This alveolar-like tissue was homogeneously distributed across all three experimental groups (53.4%, 46.7% and 40% in G0, G7 and G14, respectively) (Table 3). We then performed an IHQ test after FISH in those samples with alveolar-like tissue patches. These cells demonstrated positivity for CrY and pulmonary surfactant-associated protein B proving that these BMSCs had developed surfactant-producing properties.
Presence of alveolar-like tissue. Thick black box represents number and percentage of animals with positive FISH and IHQ among sections with presence of alveolar-like tissue.
G0: animals receiving BMSC the same day of tracheal grafting.
G7: animals receiving BMSC 7 days after tracheal grafting.
G14: animals receiving BMSC 14 days after tracheal grafting.
FISH: fluorescence in situ hybridization.
IHQ: immunohistochemistry.
Bold values highlight the most extreme and opposite values in the comparison between subgroups and control group.
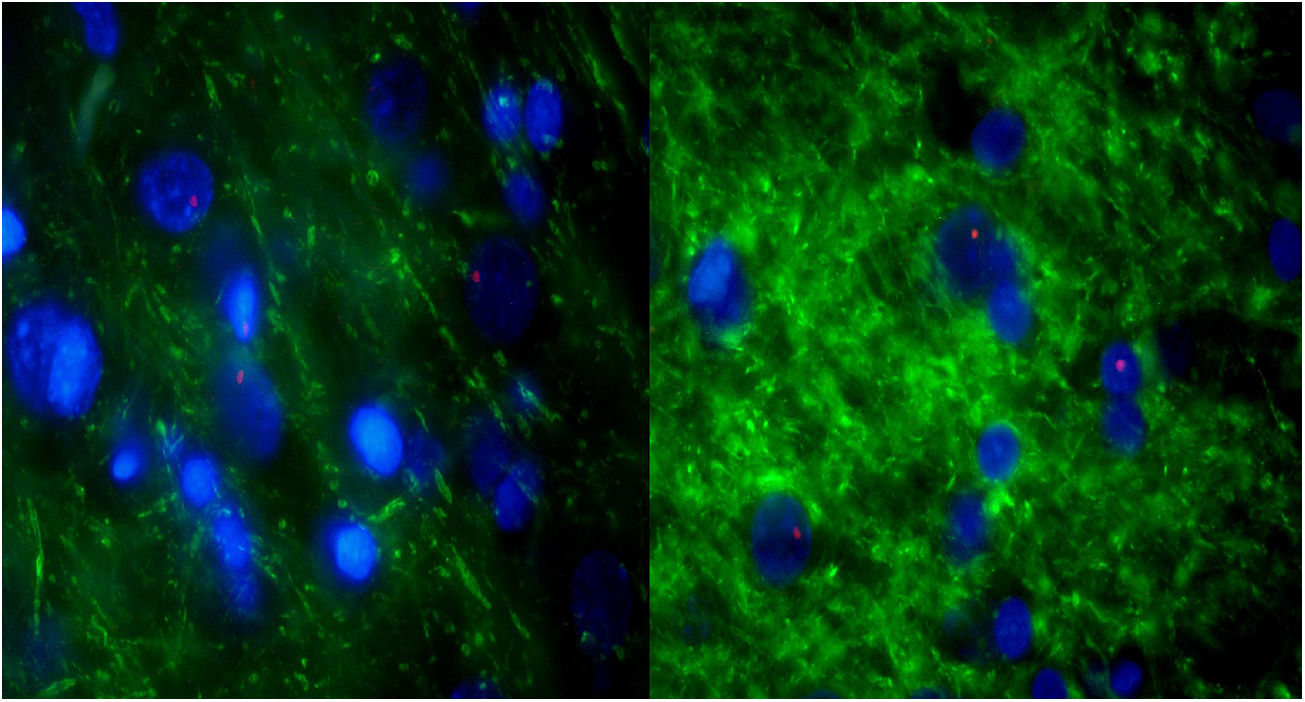
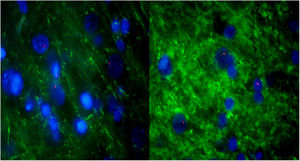
For further characterization, IHQ test after FISH was performed in those samples with alveolar-like tissue patches to clarify whether these cells were producing typical pneumocyte-type II markers (in particular, pulmonary surfactant-associated protein B). The majority of the analyzed samples showed both FISH and IHQ positiveness in the same section (Table 3). In other words, CrY+ cells (BMSC) were expressing pulmonary surfactant-associated protein B (Fig. 5).
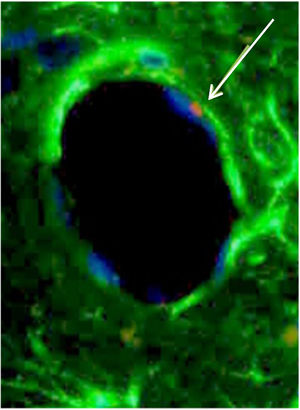
Consecutive sections of the previously described alveolar-like tissue in which we performed both FISH and IHQ (using antibodies against protein B of pulmonary surfactant) for characterization of the present cells. Under the fluorescence microscope (40×), red color indicates Sry region (CrY), nuclei can be seen in blue, and green represents protein B expression. As it can be observed, BMSCs are present in the alveolar-like tissue and they are producing protein B of pulmonary surfactant. Cells without Sry region marked with red, might be either BMSC or other cells whose differentiation might have been induced by BMSC.
CrY+ cells (BMSC) were detected in all three experimental groups (G0, G7, G14), irrespective of the transplantation route (ET, EV, LP). Also, we randomly investigated the expression of Ki67 in all groups, finding expression in all groups.
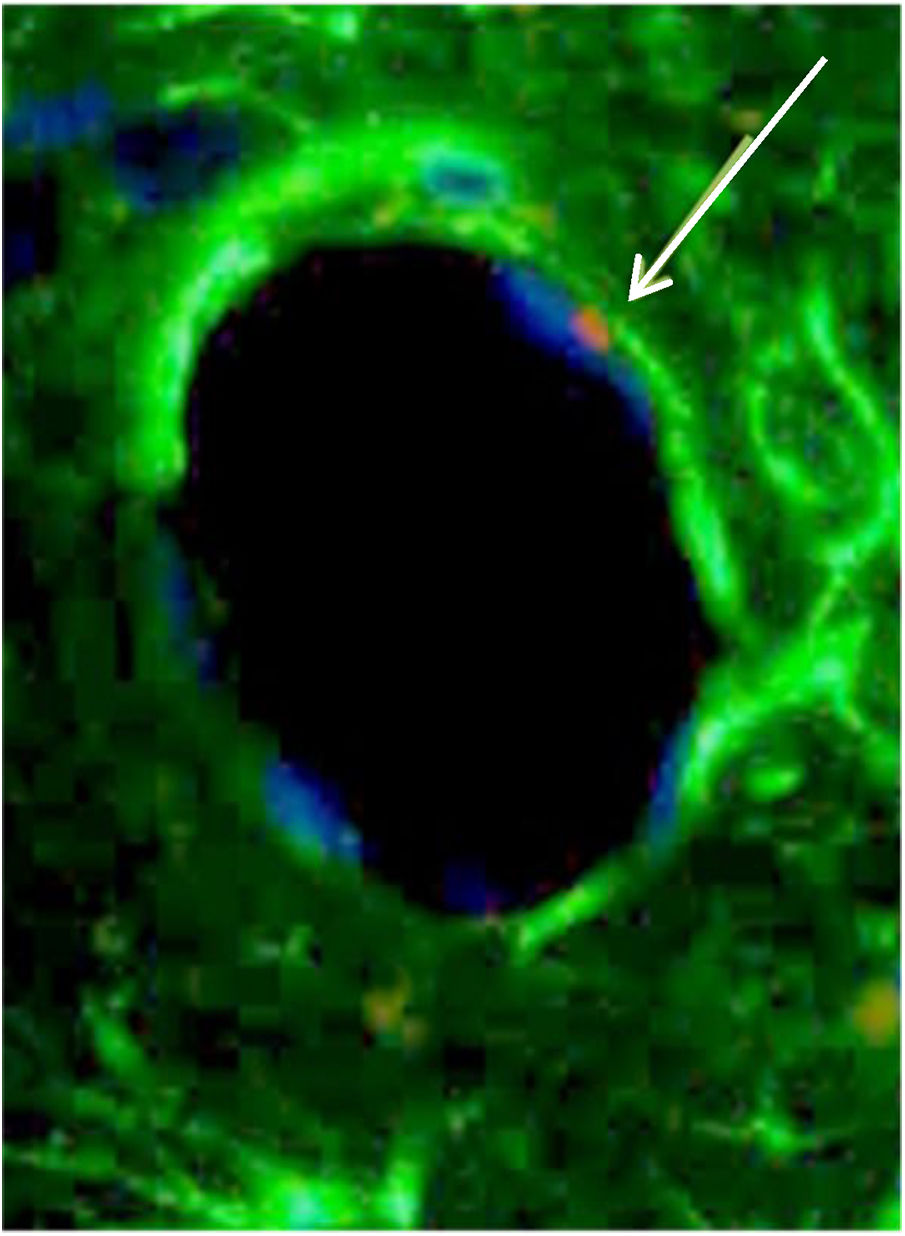
From a morphological standpoint, BMSC were observed as a part of cartilage, connective tissue and endothelium of neovessels (Fig. 6).
BMSC differentiated in an endothelial cell. White arrow is pointing to Sry region of CrY, which is marked in red in the FISH technique. Phenotypically, this BMSC (white arrow) is similar to an endothelial cell and is taking part of the structure of the vessel wall. The blue color is the nucleus, and green is connective tissue.
BOS hampers the long-term survival of lung transplant recipients. Regardless of the immunosuppressive regimen, only one-third of our patients will remain free from BOS 10 years after transplantation. Therefore, it is of paramount importance to find therapeutic strategies that prevent or attenuate the incidence of this condition.
Histopathologically, end-stage BOS consists of an excessive fibroproliferative thickening of the bronchiolar walls in response to epithelial injury. In the initial stages, lymphocytic infiltrates invade the epithelium and submucosal layer and the epithelium loses its architectural consistency. As the disease progresses, the bronchiolar lumen is progressively replaced by granulation tissue containing fibroblasts and myofibroblasts, and finally forms a fibrotic scar.3 Although this process was initially thought to be a direct result of T-cell mediated injury of graft structures,17 there is now evidence to support that antibody-mediated rejection (circulating antibodies to donor HLA molecules)18,19 and autoimmune responses to airway components (collagen V, Kα-1 tubulin) are also associated.20,21
Heterotopic tracheal transplantation into the lung parenchyma has been successfully used to reproduce the histologic changes of BO lesion and investigate angiogenesis and fibroproliferative tissue remodeling.14 However, some limitations have been described with regard to this animal model, such as the lack of connection of the graft to the airway or vessels, as well as the fact that BO occurs in the small airways in the clinical setting. Despite these limitations, we were able to reproduce the typical histological changes of BO lesions.
Our results show that BMSC are able to impact the histologic course of BOS at any time point and through any route of administration, moving from a pattern of dense fibrous scar tissue to another kind of histologic presentation, with various degrees of inflammation, edema and neovascularization.
The most impressive reductions in fibrosis were seen when BMSC were applied at day 7. In particular, the rats receiving BMSC via the endotracheal route at day 7 showed no fibrosis and no inflammation at all, suggesting that early endobronchial therapy should be the preferred choice.
Concerning the dosage of BMSC, the literature is unclear on its importance.10,12 The most effective concentration has not yet been established and warrants further research. Therefore, we planned to use the maximum tolerated concentration depending on the route of administration.
It has been described, both in vitro and in vivo, that mesenchymal stem cells (MSC) have immunomodulatory properties that promote or prevent innate and acquired inflammatory response, depending on the local environment. They can inhibit the proliferation and function of T-Lymphocytes, natural killer (NK) cells and dendritic cells (DC). MSC are able to prompt T cell expansion toward a regulatory phenotype (Treg) by the production of prostaglandin E2 (PGE2) and transforming growth factor β-1 (TGFβ-1).22 Similarly, MSC can shift the macrophage population toward a predominantly immunosuppressive M2 phenotype by secretion of IL-10, IL-2 and IL-13.23,24 The effect of MSC in B cells is controversial; some studies have shown inhibited proliferation, differentiation and immunoglobulin production,25–27 whereas other groups have reported stimulatory effects.28,29
The differentiation of BMSCs is strongly influenced by T cell activation and the type and concentrations of cytokines in the local environment at the time of the exposure, which explains the different properties they can show in different scenarios.30
This special feature of MSC supports our observation that the administration of these cells at the appropriate moment is critical to get the desired effect. The most impressive results were seen at day 7, when histopathological changes were at early stages in our model, with some degree of inflammatory activity. Prior to this (T0), there was no damage to restore and, later on (T14), established fibrotic changes exceeded their ability to completely reverse the process.
When tissue is damaged, circulating MSC are attracted to the site, and, as they have multipotent differentiation potential, they can differentiate into mature functional cells replacing damaged ones. Nonetheless, this is arguably a minor event, as MSC mainly exert their reparative effects by stimulating in situ progenitor cells, endothelial cells and fibroblasts to repair the injury through immunoregulation and growth factor secretion (i.e., paracrine mechanism).31
One of the most interesting findings in our experiment is the BMSC-mediated response to injury, developing an alveolar-like tissue and mimicking what is supposed to be a normal tissue in that environment. Moreover, those cells involved in this peculiar tissue showed BMSC markers and produced surfactant protein B, a specific biomarker of alveolar epithelial cells. Although it is impossible to proof whether this alveolar-like tissue is functional or not, this finding not only shows BMSC inclination to repair and restore normal tissue, but also it might be proof of their transdifferentiation ability,31–33 leading to mature cells of different lineages.
Another example of this is the finding of BMSC (CrY+) taking part of the walls of neovessels, which are morphologically identical to endothelial cells (Fig. 5).
Previous heterotopic tracheal transplant models have shown the capacity of stem cells to attenuate histopathological changes of BO after either topical or systemic administration.34–36 In this study, we have found that BMSC are able to nest and survive, detect an injury scenario, modify the histopathological course of BO lesions, and repair the structure. Moreover, although intravenous and locally injected BMSC have an impact in the BO lesions, the most remarkable effects are seen after endotracheal instillation of BMSC. This finding might mean that the endoscopic administration of BMSC should be considered for further experimental or clinical projects, while potential systemic collateral effects in other organs are minimized.
Given the fact that lung injury and repair is a complex process in a model of BOS, it is difficult to attribute our experimental results to one or another mechanism led by BMSC. It is clear that both paracrine, as the main mechanism, and differentiation, as a minor one, have a role in the modification of the histological course of BOS.
Although we have been able to find statistically significant results with very small sample sizes, using a control group and masking techniques, we must be cautious and await the results of this type of therapy in other models before generalizing our findings.
In the clinical scenario, there have been two phase I studies so far. Chambers et al.37 infused 2×106 BMSC from an unrelated donor to 10 patients with BOS via a peripheral vein twice a week for two weeks, without any procedure related toxicity. Similarly, Keller et al.38 infused 1–4 million of allogeneic mesenchymal stem cells into nine patients with moderate BOS without any negative impact in measured clinical, functional or laboratory variables, opening the door for the use of these cells in the setting of established BOS.
ConclusionsBone marrow-derived mesenchymal stem cells modify the histopathological development of BO in this heterotopic tracheal transplantation animal model of BO. Moreover, they are able to express alveolar cell markers, such us pulmonary surfactant protein B.
Endotracheal administration of BMSCs might be considered an effective way of preventing BO development in this animal model.
FundingThis project has been granted by Fundación Mutua Madrileña (identification number: PR87532011).
AuthorshipDavid Gomez-de-Antonio: design, animal surgeries, interpretation of results, manuscript.
Jose Luis Campo-Cañaveral de la Cruz: design, experimental surgeries, interpretation of results, manuscript.
Mercedes Zurita: FISH and IHQ techniques.
Martín Santos: experimental surgeries anesthesia.
Carmen González Lois: pathological analysis of the samples.
Andrés Varela: manuscript edition.
Jesús Vaquero: manuscript edition.
Conflict of interestsThe authors declare no conflict of interests.