Acute respiratory distress syndrome (ARDS) is still associated with high mortality despite the considerable efforts devoted to improving its treatment from the perspectives of basic science and clinical research. Cell therapy was proposed as a potential new tool for treating ARDS and the results obtained so far from preclinical research are encouraging.1 Mesenchymal stem (stromal) cells (MSCs) are particularly interesting for this application, not because of their potential differentiation into lung cell phenotypes, but because of their ability to release agents (e.g., paracrine factors, microvesicles, mitochondria) with immunomodulatory, anti-inflammatory and antimicrobial effects.2–4 The promising results obtained using MSCs in animal and ex vivo human lung ARDS models provided background to launch the first clinical trials which have recently finished or are still in progress.5,6 However, determining the technical details (e.g. cell origin and preparation, administration procedure and dosage) to optimize the potential therapeutic effects of MSCs in ARDS is still an open issue.1
Preconditioning MSCs before their application to patients could be relevant since it would pre-activate repair physiological pathways in these cells. It is known that modifying the microenvironment of MSCs modulates their paracrine signalling.7 In particular, MSCs sense and actively respond to their biophysical microenvironment. For example, secretion of a wide range of cytokines is regulated by the stiffness of MSCs microenvironment8 and stretching enhances angiogenic and anti-apoptotic capacities in these cells.9,10 The fact that MSCs exhibit such responses to biophysical stimuli is particularly interesting for treating ARDS since cells in the target organ are placed on microenvironments with different stiffness11 and undergo continuous mechanical stretching owing to ventilation. Therefore, we hypothesized that preconditioning MSCs by subjecting them to conditions realistically mimicking the biophysical microenvironment in the lung would improve their effectiveness in the treatment of ARDS. Here we describe a proof of concept test of this hypothesis. The study (approved by the Institutional Ethics Board) was carried out on a rat model (Sprague Dawley, male, 200–300g) of ventilator-induced lung injury (VILI). Specifically, we biophysically preconditioned MSCs by culturing the cells on lung extracellular matrix (ECM) to expose them to realistic biochemical and stiffness substrate cues and by simultaneously subjecting MSCs to cyclical stretch simulating ventilation.
Lung-derived MSCs were obtained from Sprague Dawley rats12 weighing 200–300g and conventionally characterized by their cell surface markers (CD29, CD44H, CD45, CD11b/c and CD90) and differentiation capability (adipocytes, osteocytes and chondrocytes). Moreover, 70-μm slices of lung ECM obtained by decellularizing rat lungs11 were attached on the flexible membrane of computer-controlled polydimethylsiloxane (PDMS) chip specifically designed to subject cells to frequency- and amplitude-controlled dynamic stretch by applying cyclical positive pressure underneath the membrane.13 Subsequently, MSCs were mechanically preconditioned by seeding them on the lung ECM slices and subjected to cyclic stretch mimicking lung ventilation (20% amplitude, 12cycles/min) for 7 days. Additionally, non-preconditioned MSCs were cultured in parallel in conventional flasks.
Both MSCs with/out preconditioning were used to treat VILI induced in anesthetized, tracheostomized and paralyzed Sprague Dawley rats initially subjected to baseline ventilation (7mL/kg, PEEP=3cmH2O, 70cycles/min, 21% O2) and then subjected to injurious ventilation (35cmH2O inspiratory pressure, zero PEEP) until achieving an increase of ∼20% in respiratory elastance, which would induce a relatively mild VILI.14 Injurious ventilation time was 103±18min (m±SD), with no significant difference among groups (P=.665, One Way ANOVA). After this time point, baseline ventilation was resumed for 30min and treatment with MSCs was applied by femoral venous injection. To this end, rats with VILI were randomly distributed into 3 treatment groups (N=8 each): vehicle, non-preconditioned MSCs and preconditioned MSCs (4×106cells/kg in 500μL, in both cases). After treatment, the rats were kept under baseline ventilation for 4h and then (end-point) elastance was measured, bronchoalveolar lavage fluid (BALF) was obtained from one lung for measuring total cell, neutrophil, protein and inflammatory cytokines (TNF-α and CXCL2) concentrations, and the other lung was excised for assessing oedema by its weight/dry ratio.15 A group of control rats (N=8) was maintained under initial ventilation (no VILI, no treatment) for the same total time. MSCs engraftment in the lungs was assessed in additional VILI rats treated with fluorescently (PKH26) stained MSCs with/out (N=6 each) preconditioning.
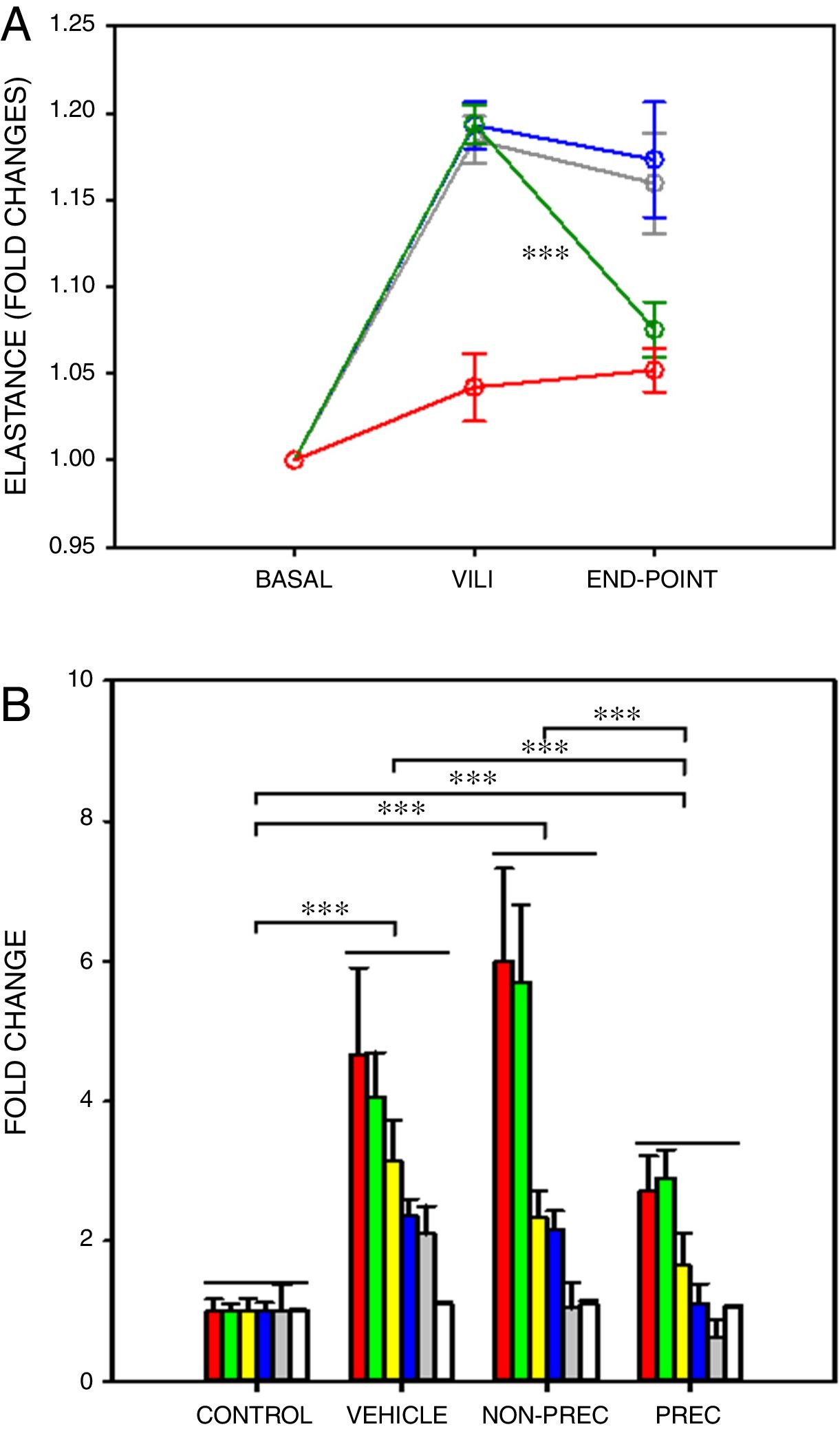
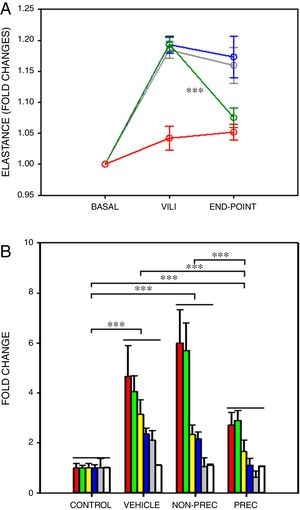
After a ∼20% increase in elastance, established by protocol, only preconditioned MSCs induced a significant recovery in elastance (Fig. 1A; paired t-test). In VILI, lung oedema increased by 16% and all the other 6 outcome variables augmented by more than 2-fold (Fig. 1B), reflecting typical increase in alveolar-capillary membrane permeability and inflammation. The statistical significance of the overall effect of VILI treatment with MSCs was assessed by conducting a rank-ANOVA to the sum of the 6 outcome variables after applying a normal standardization (Fig. 1B). The novel and relevant result of this study, which confirms our hypothesis, was that biophysically preconditioned MSCs were more effective than non-preconditioned MSCs in reducing VILI. In fact, while the effect of non-preconditioned MSCs was low, preconditioned MSCs achieved a significant recovery from VILI (Fig. 1B). This improvement in treatment was not associated with a significant difference in MSCs engraftment into the lungs (P=.997, t-test).
Biophysically preconditioned MSCs reduced VILI. (A) Time course of respiratory elastance (relative to baseline value) in rats subjected to VILI and treated with: vehicle (no-MSCs) (grey), non-preconditioned MSCs (blue) and preconditioned MSCs (green). Red line corresponds to rats maintained with baseline ventilation (no VILI induction). *** Means P<.001 comparing elastance measured after 30min of injured ventilation (VILI) and after 4h of treatment (END-POINT) with paired t-test. (B) Fold changes measured at the END-POINT in lung oedema (white) and bronchoalveolar lavage fluid concentrations of proteins (green), total cells (red), neutrophils (grey), TNF-α (blue) and CXCL2 (yellow), for control ventilation (CONTROL: no VILI induction) and treatment with: VEHICLE (no-MSCs), non-preconditioned (NON-PREC) MSCs and preconditioned (PREC) MSCs. Data are mean±SE. *** Means P<.001 in a rank-ANOVA to the sum of these 6 variables after applying a normal standardization.
This is the first study providing support to the novel notion that biophysically preconditioning MSCs could optimize the therapy in ARDS. However, like any proof of concept test, our study has limitations since lung histology, gas exchange, systemic inflammation and repair mechanisms potentially involved (e.g. keratinocyte growth factor14) were not assessed. Notwithstanding, this work opens a considerable number of important questions. Among the more specific, whether the positive effects of biophysically preconditioned MSCs we observed would be reproduced in a more severe and long-term VILI model. More general questions include the comparison of the effects of preconditioning lung-derived vs bone marrow-derived MSCs, or application of MSCs vs their supernatant, extracellular vesicles or mRNAs. It would also be important to test the effects of biophysically preconditioned MSCs on other experimental one-hit or two-hit models of intra- and extra-pulmonary ARDS (e.g. induced by LPS, bacterial lung infection, acid lavage, intra-abdominal or systemic sepsis).
In conclusion, we believe that this study opens an avenue for future research aimed at understanding the basic repair mechanisms activated in MSCs by physiomimetic stimuli, with the translational perspective towards the use of these cells as a tool to improve the treatment of lung injury.
FundingThis work was supported in part by the Spanish Ministry of Economy and Competitiveness (SAF2017-85574-R, DPI2017-83721-P) and Generalitat de Catalunya (Programa CERCA). PNN was supported by National Counsel of Technological and Scientific Development from Brazil – CNPq (207258/2014-7).
The authors wish to thank Dr. Ferran Torres, Medical Statistics Core Facility, Institut Investigacions Biomediques August Pi Sunyer, for his support in the statistical analysis of the data.