The main aim of this international consensus document on obstructive sleep apnea is to provide guidelines based on a critical analysis of the latest literature to help health professionals make the best decisions in the care of adult patients with this disease. The expert working group was formed primarily of 17 scientific societies and 56 specialists from a wide geographical area (including the participation of 4 international societies), an expert in methodology, and a documentalist from the Iberoamerican Cochrane Center. The document consists of a main section containing the most significant innovations from the ICD and a series of online manuscripts that report the systematic literature searches performed for each section of the ICD. This document does not discuss pediatric patients or the management of patients receiving chronic non-invasive mechanical ventilation (these topics will be addressed in separate consensus documents).
El objetivo principal de este documento internacional de consenso sobre apnea obstructiva del sueño es proporcionar unas directrices que permitan a los profesionales sanitarios tomar las mejores decisiones en la asistencia de los pacientes adultos con esta enfermedad según un resumen crítico de la literatura más actualizada. El grupo de trabajo de expertos se ha constituido principalmente por 17 sociedades científicas y 56 especialistas con amplia representación geográfica (con la participación de 4 sociedades internacionales), además de un metodólogo experto y un documentalista del Centro Cochrane Iberoamericano. El documento consta de un manuscrito principal, con las novedades más relevantes del DIC, y una serie de manuscritos online que recogen las búsquedas bibliográficas sistemáticas de cada uno de los apartados del DIC. Este documento no cubre la edad pediátrica ni el manejo del paciente en ventilación mecánica crónica no invasiva (que se publicarán en sendos documentos de consenso aparte).
The main objective of this international consensus document (ICD) on obstructive sleep apnea (OSA) is to provide guidelines based on a critical analysis of the latest literature to help healthcare professionals make the best decisions in the care of adult patients with this disease. This document does not discuss pediatric patients or the management of patients receiving chronic non-invasive mechanical ventilation (these topics will be addressed in separate consensus documents).
The task force was formed primarily of 17 scientific societies and 56 specialists from a wide geographical area (including 4 international societies), an expert in methodology, and a documentalist from the Iberoamerican Cochrane Center, all of whom participated as consultants and conducted the systematic literature search.
The literature search strategy was primarily designed to identify systematic reviews published in the last 10 years in English or Spanish, followed by randomized clinical trials, observational studies, clinical practice guidelines, and economic studies according to the topic of each section. Validated methodological filters were used to identify the different types of study design.
The search was conducted on MEDLINE (via PubMed), EMBASE (via Ovid), The Cochrane Library, and CENTRAL (Appendix B available in online material).
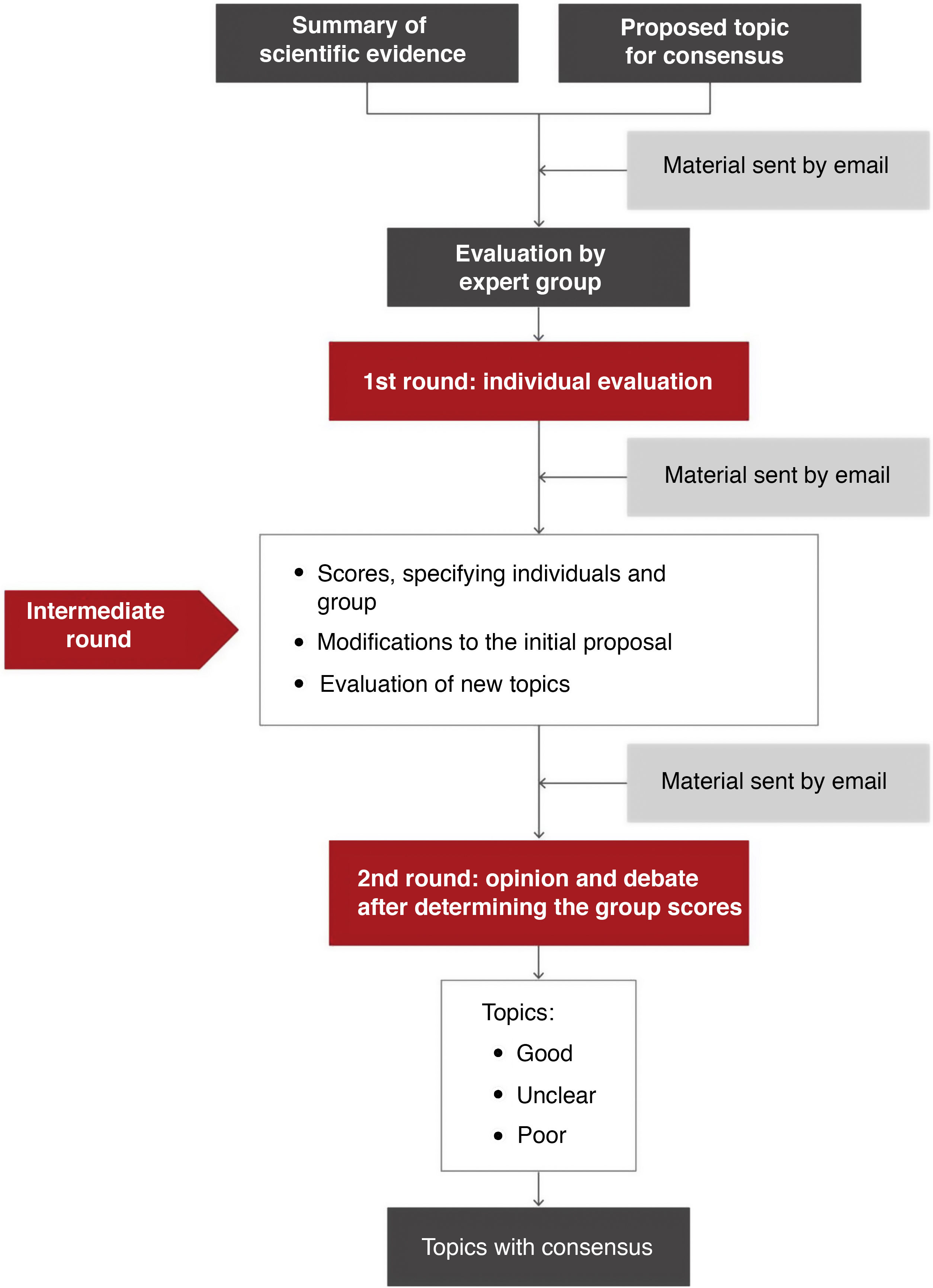
MethodologyAn adaptation of the RAND-UCLA method1,2 was used for the preparation of this document. The responsible organizations and the general coordinator of the project established the topics for consensus and selected the experts and task force leaders.
Topics requiring a systematic search of the scientific literature were identified. Structured searches were conducted by expert documentalists. The document was subsequently developed from a draft drawn up by the leaders of each task force. The experts in each group expressed their agreement on the areas for consensus (Table 1 and Fig. 1).
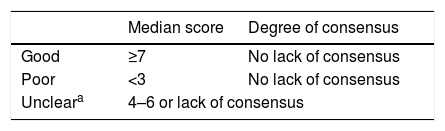
Classification of the degree of consensus on topics included.
| Median score | Degree of consensus | |
|---|---|---|
| Good | ≥7 | No lack of consensus |
| Poor | <3 | No lack of consensus |
| Uncleara | 4–6 or lack of consensus | |
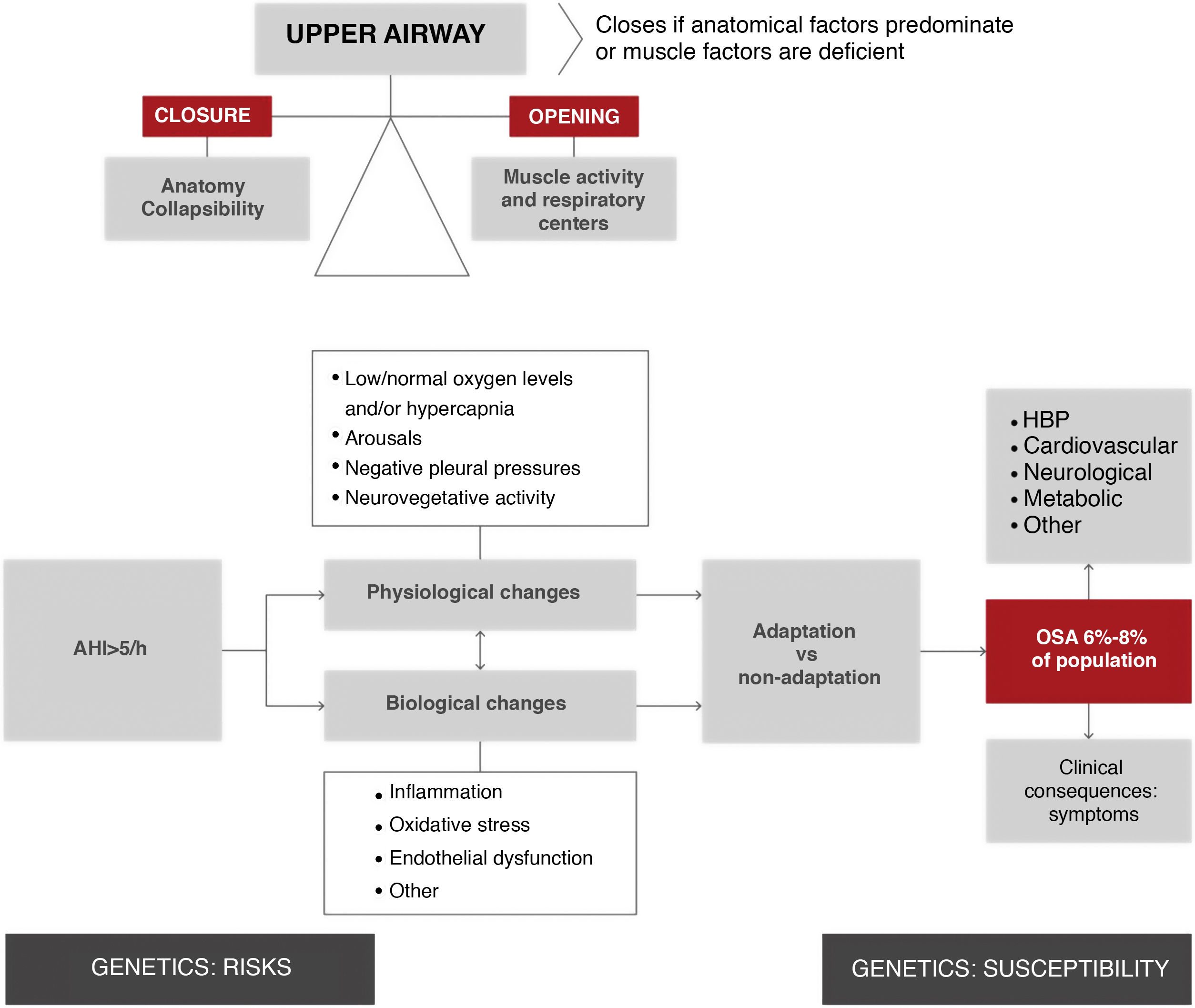
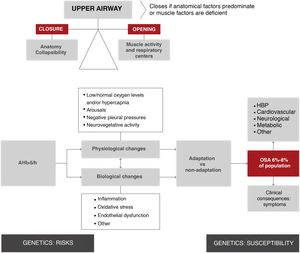
The upper airway of patients with obstructive apneas tends to collapse during sleep, resulting in total or partial airway occlusion. Breathing stops until a microarousal occurs, which reactivates the muscles and reopens the airway. Apnea occurs when the factors that tend to close the airway cannot be offset by the ability of the dilating muscles of the pharynx and/or respiratory centers to keep it open (Fig. 2).
Overview of the physiological and biological processes of obstructive sleep apnea. As shown in the top left section of the figure, upper airway obstruction is the result of an imbalance between forces that tend to keep it open (muscle activity) and forces that tend to close it (anatomical factors). This imbalance increases the collapsibility of the upper airway, resulting in the respiratory episode (apnea hypopnea). It is estimated that 19% of the general population has an apnea-hypopnea rate > 10/h3. These episodes involve a series of physiological changes (hypoxia, transient arousals, and intrathoracic pressure changes) and biological changes (inflammation, oxidative stress, etc.). Depending on individual adaptation phenomena, these episodes cause secondary disease in the form of symptoms and are risk factors for the development of various entities (HBP, among others). Several generic factors modulate predisposition to these consequences. AHI: apnea-hypopnea index; HBP: high blood pressure; OSA: obstructive sleep apnea.
This ICD proposes updating the nomenclature of sleep apnea syndrome to reintroduce the term “obstructive” in the acronyms accepted in 2005, since it defines the nature of the disease and clearly differentiates it from central sleep apnea. We decided to simplify the nomenclature and to remove the word “hypopnea” and the word “syndrome”, which is an outdated term that fails to reflect the current reality of the disease. We therefore recommended the use of the term “obstructive sleep apnea” and the acronym “OSA”.
In this ICD, OSA is defined as the presence of one of the following criteria:
- 1
Presence of an apnea-hypopnea index (AHI) ≥ 15/h that is predominantly obstructive.
- 2
AHI ≥ 5/h accompanied by one or more of the following factors: excessive daytime sleepiness, non-restorative sleep, excessive tiredness, and/or impaired sleep-related quality of life, which cannot be explained by other causes.
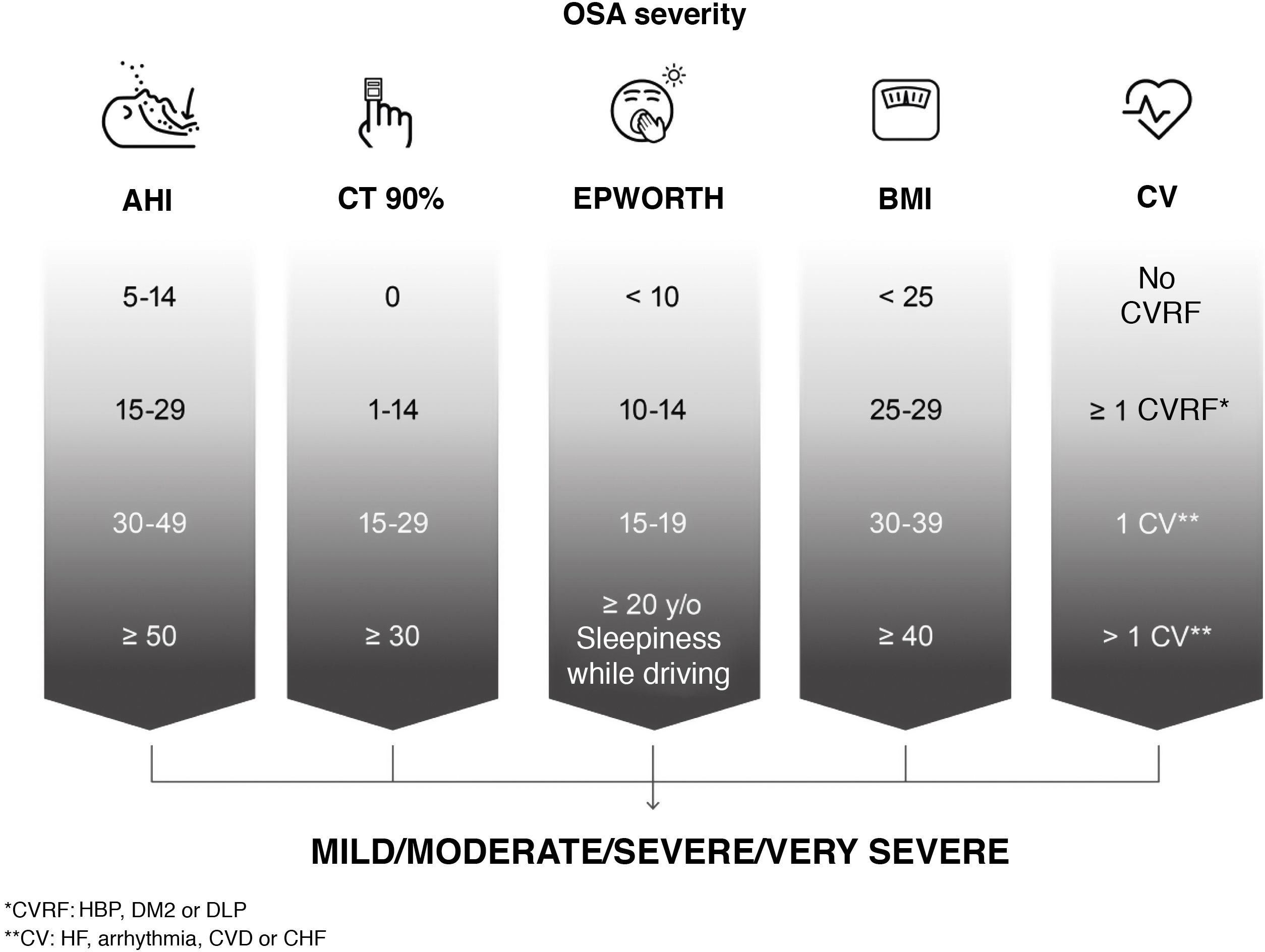
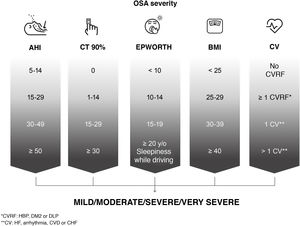
For the assessment of OSA severity, the position of this ICD is that classifications based solely on AHI are limited and do not reflect the heterogeneity of the disease. In line with current thinking among the scientific community4,5, we have prioritized the search for new scores that reflect this heterogeneity and are predictors of the long-term effects of the disease. Since no validated score is currently available, and although the factors or cut-off points leading to a classification of severe are not clearly established, we recommend that the following be taken into account: AHI; time with oxygen saturation below 90%, reflecting hypoxemia; daytime sleepiness; degree of obesity measured by body mass index and comorbidities (risk factors and cardiovascular disease) associated with OSA (high blood pressure [HBP], especially if treatment-refractory or non-dipping; diabetes mellitus type 2 [DM2]; dyslipidemia; coronary disease; stroke; heart failure or atrial fibrillation) (Fig. 3).
Assessment of the severity of the patient with obstructive sleep apnea (OSA) based on various objective parameters recommended by this International Consensus Document. AHI: apnea-hypopnea index; HBP: high blood pressure; BMI: body mass index; CHF: congestive heart failure; CT 90%: accumulated time with oxygen saturation below 90%; CV: cardiovascular or cerebrovascular disease; CVD: cerebrovascular disease; CVRF: cardiovascular risk factors; DLP: dyslipidemia; DM2: diabetes mellitus type 2; EPWORTH: Epworth Sleepiness Scale; IHD: ischemic heart disease.
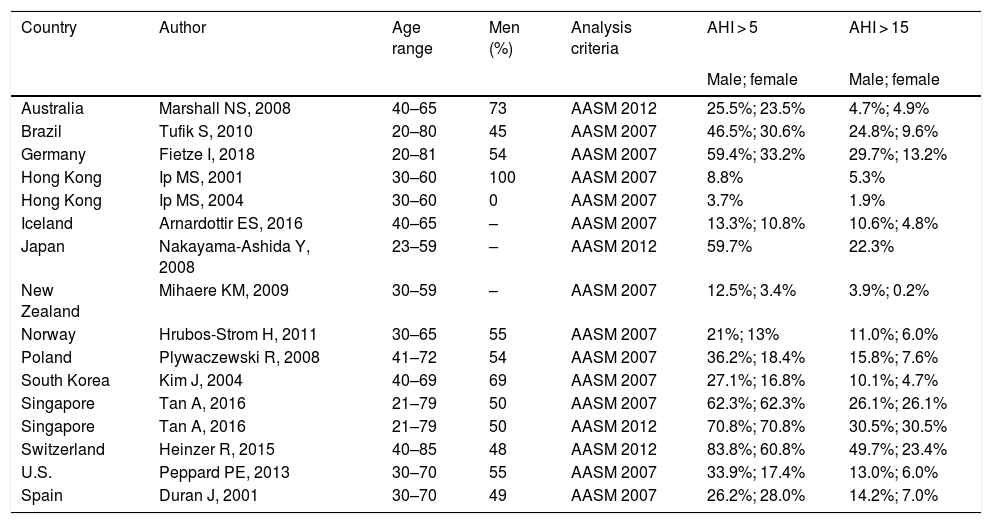
OSA is one of the most prevalent sleep disorders, but epidemiological studies in the literature vary widely in terms of methodology, the inclusion of clinical series or population series, diagnostic criteria, and the assessment of severity. A recently published study that analyzed the global burden of this entity6 reported rates ranging between 4% and 30%. Results are summarized in Table 2.
Studies reporting country-specific OSA prevalence data.
| Country | Author | Age range | Men (%) | Analysis criteria | AHI > 5 | AHI > 15 |
|---|---|---|---|---|---|---|
| Male; female | Male; female | |||||
| Australia | Marshall NS, 2008 | 40–65 | 73 | AASM 2012 | 25.5%; 23.5% | 4.7%; 4.9% |
| Brazil | Tufik S, 2010 | 20–80 | 45 | AASM 2007 | 46.5%; 30.6% | 24.8%; 9.6% |
| Germany | Fietze I, 2018 | 20–81 | 54 | AASM 2007 | 59.4%; 33.2% | 29.7%; 13.2% |
| Hong Kong | Ip MS, 2001 | 30–60 | 100 | AASM 2007 | 8.8% | 5.3% |
| Hong Kong | Ip MS, 2004 | 30–60 | 0 | AASM 2007 | 3.7% | 1.9% |
| Iceland | Arnardottir ES, 2016 | 40–65 | – | AASM 2007 | 13.3%; 10.8% | 10.6%; 4.8% |
| Japan | Nakayama-Ashida Y, 2008 | 23–59 | – | AASM 2012 | 59.7% | 22.3% |
| New Zealand | Mihaere KM, 2009 | 30–59 | – | AASM 2007 | 12.5%; 3.4% | 3.9%; 0.2% |
| Norway | Hrubos-Strom H, 2011 | 30–65 | 55 | AASM 2007 | 21%; 13% | 11.0%; 6.0% |
| Poland | Plywaczewski R, 2008 | 41–72 | 54 | AASM 2007 | 36.2%; 18.4% | 15.8%; 7.6% |
| South Korea | Kim J, 2004 | 40–69 | 69 | AASM 2007 | 27.1%; 16.8% | 10.1%; 4.7% |
| Singapore | Tan A, 2016 | 21–79 | 50 | AASM 2007 | 62.3%; 62.3% | 26.1%; 26.1% |
| Singapore | Tan A, 2016 | 21–79 | 50 | AASM 2012 | 70.8%; 70.8% | 30.5%; 30.5% |
| Switzerland | Heinzer R, 2015 | 40–85 | 48 | AASM 2012 | 83.8%; 60.8% | 49.7%; 23.4% |
| U.S. | Peppard PE, 2013 | 30–70 | 55 | AASM 2007 | 33.9%; 17.4% | 13.0%; 6.0% |
| Spain | Duran J, 2001 | 30–70 | 49 | AASM 2007 | 26.2%; 28.0% | 14.2%; 7.0% |
AASM: American Academy of Sleep Medicine; AHI: apnea-hypopnea index.
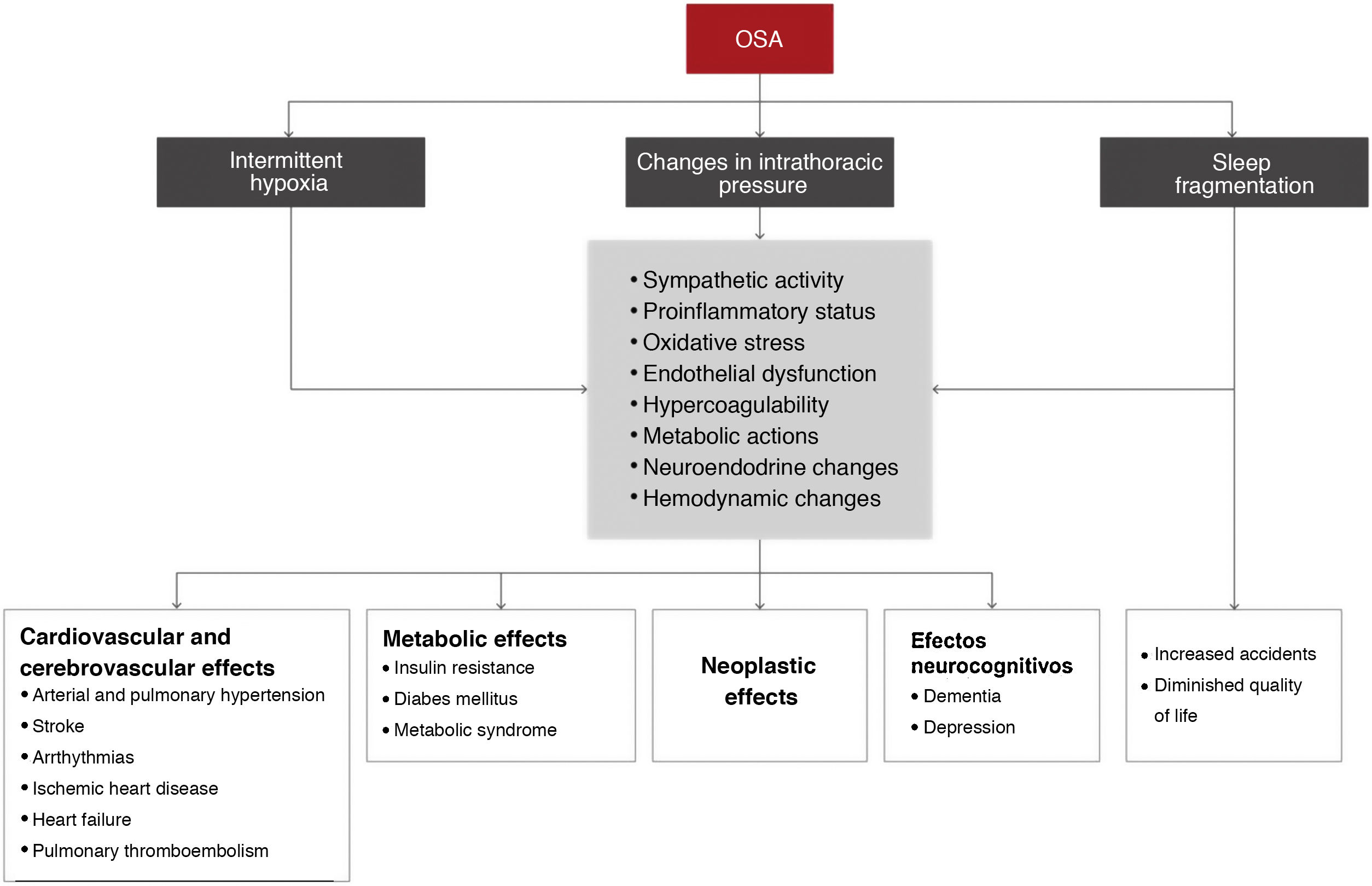
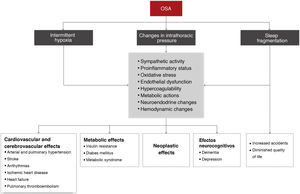
The main pathophysiological mechanisms that underlie the association between OSA and its consequences are intermittent hypoxia, sleep fragmentation, intrathoracic pressure changes, and a number of intermediate elements (Fig. 4).
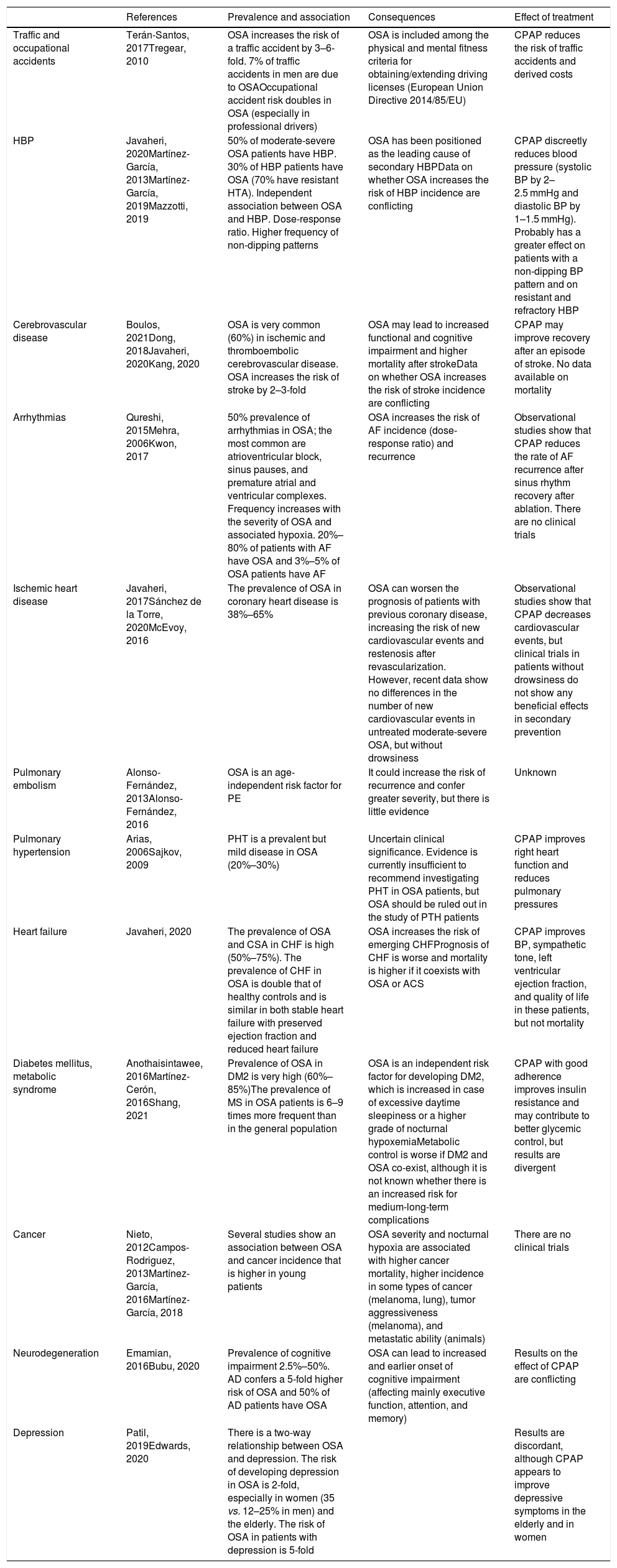
Table 3 summarizes the scientific evidence available on the association between OSA and its different consequences. OSA increases the risk of workplace and road traffic accidents. In the cardiovascular field, one of the manifestations for which the most evidence is available is arterial hypertension. The prevalence of OSA in DM2 is very high and OSA is an independent risk factor for developing DM2. OSA is also very common in coronary disease, but data on its clinical consequences and the effect of treatment are conflicting. OSA also increases the risk of stroke and may be associated with greater functional/cognitive impairment and higher mortality. Sleep-disordered breathing in heart failure is very prevalent, increases the risk of new episodes, and may increase mortality. Arrthythmias, pulmonary thromboembolism, and pulmonary arterial hypertension are also associated with a very high rate of OSA. Severe OSA is also associated with an increased risk of cancer, mortality, and tumor aggressiveness (melanoma), although current evidence is not yet strong. In neurocognitive terms, OSA causes major cognitive impairment (mainly in executive function, attention, and memory) and has a bidirectional association with depression.
Consequences of obstructive sleep apnea: prevalence, incidence, consequences and effect of treatment.
| References | Prevalence and association | Consequences | Effect of treatment | |
|---|---|---|---|---|
| Traffic and occupational accidents | Terán-Santos, 2017Tregear, 2010 | OSA increases the risk of a traffic accident by 3–6-fold. 7% of traffic accidents in men are due to OSAOccupational accident risk doubles in OSA (especially in professional drivers) | OSA is included among the physical and mental fitness criteria for obtaining/extending driving licenses (European Union Directive 2014/85/EU) | CPAP reduces the risk of traffic accidents and derived costs |
| HBP | Javaheri, 2020Martínez-García, 2013Martínez-García, 2019Mazzotti, 2019 | 50% of moderate-severe OSA patients have HBP. 30% of HBP patients have OSA (70% have resistant HTA). Independent association between OSA and HBP. Dose-response ratio. Higher frequency of non-dipping patterns | OSA has been positioned as the leading cause of secondary HBPData on whether OSA increases the risk of HBP incidence are conflicting | CPAP discreetly reduces blood pressure (systolic BP by 2–2.5 mmHg and diastolic BP by 1–1.5 mmHg). Probably has a greater effect on patients with a non-dipping BP pattern and on resistant and refractory HBP |
| Cerebrovascular disease | Boulos, 2021Dong, 2018Javaheri, 2020Kang, 2020 | OSA is very common (60%) in ischemic and thromboembolic cerebrovascular disease. OSA increases the risk of stroke by 2–3-fold | OSA may lead to increased functional and cognitive impairment and higher mortality after strokeData on whether OSA increases the risk of stroke incidence are conflicting | CPAP may improve recovery after an episode of stroke. No data available on mortality |
| Arrhythmias | Qureshi, 2015Mehra, 2006Kwon, 2017 | 50% prevalence of arrhythmias in OSA; the most common are atrioventricular block, sinus pauses, and premature atrial and ventricular complexes. Frequency increases with the severity of OSA and associated hypoxia. 20%–80% of patients with AF have OSA and 3%–5% of OSA patients have AF | OSA increases the risk of AF incidence (dose-response ratio) and recurrence | Observational studies show that CPAP reduces the rate of AF recurrence after sinus rhythm recovery after ablation. There are no clinical trials |
| Ischemic heart disease | Javaheri, 2017Sánchez de la Torre, 2020McEvoy, 2016 | The prevalence of OSA in coronary heart disease is 38%–65% | OSA can worsen the prognosis of patients with previous coronary disease, increasing the risk of new cardiovascular events and restenosis after revascularization. However, recent data show no differences in the number of new cardiovascular events in untreated moderate-severe OSA, but without drowsiness | Observational studies show that CPAP decreases cardiovascular events, but clinical trials in patients without drowsiness do not show any beneficial effects in secondary prevention |
| Pulmonary embolism | Alonso-Fernández, 2013Alonso-Fernández, 2016 | OSA is an age-independent risk factor for PE | It could increase the risk of recurrence and confer greater severity, but there is little evidence | Unknown |
| Pulmonary hypertension | Arias, 2006Sajkov, 2009 | PHT is a prevalent but mild disease in OSA (20%–30%) | Uncertain clinical significance. Evidence is currently insufficient to recommend investigating PHT in OSA patients, but OSA should be ruled out in the study of PTH patients | CPAP improves right heart function and reduces pulmonary pressures |
| Heart failure | Javaheri, 2020 | The prevalence of OSA and CSA in CHF is high (50%–75%). The prevalence of CHF in OSA is double that of healthy controls and is similar in both stable heart failure with preserved ejection fraction and reduced heart failure | OSA increases the risk of emerging CHFPrognosis of CHF is worse and mortality is higher if it coexists with OSA or ACS | CPAP improves BP, sympathetic tone, left ventricular ejection fraction, and quality of life in these patients, but not mortality |
| Diabetes mellitus, metabolic syndrome | Anothaisintawee, 2016Martínez-Cerón, 2016Shang, 2021 | Prevalence of OSA in DM2 is very high (60%–85%)The prevalence of MS in OSA patients is 6–9 times more frequent than in the general population | OSA is an independent risk factor for developing DM2, which is increased in case of excessive daytime sleepiness or a higher grade of nocturnal hypoxemiaMetabolic control is worse if DM2 and OSA co-exist, although it is not known whether there is an increased risk for medium-long-term complications | CPAP with good adherence improves insulin resistance and may contribute to better glycemic control, but results are divergent |
| Cancer | Nieto, 2012Campos-Rodriguez, 2013Martínez-García, 2016Martínez-García, 2018 | Several studies show an association between OSA and cancer incidence that is higher in young patients | OSA severity and nocturnal hypoxia are associated with higher cancer mortality, higher incidence in some types of cancer (melanoma, lung), tumor aggressiveness (melanoma), and metastatic ability (animals) | There are no clinical trials |
| Neurodegeneration | Emamian, 2016Bubu, 2020 | Prevalence of cognitive impairment 2.5%–50%. AD confers a 5-fold higher risk of OSA and 50% of AD patients have OSA | OSA can lead to increased and earlier onset of cognitive impairment (affecting mainly executive function, attention, and memory) | Results on the effect of CPAP are conflicting |
| Depression | Patil, 2019Edwards, 2020 | There is a two-way relationship between OSA and depression. The risk of developing depression in OSA is 2-fold, especially in women (35 vs. 12–25% in men) and the elderly. The risk of OSA in patients with depression is 5-fold | Results are discordant, although CPAP appears to improve depressive symptoms in the elderly and in women |
AD: Alzheimer's disease; AF: atrial fibrillation; HBP: high blood pressure; BMI: body mass index; BP: blood pressure; CHF: congestive heart failure; CPAP: continuous positive upper airway pressure; CSA: central sleep apnea; DM2: diabetes mellitus type 2; MS: metabolic syndrome; OSA: obstructive sleep apnea; PE: pulmonary embolism; PHT: pulmonary arterial hypertension.
Please refer to the online material for a detailed description of clinical presentation, physical examination, and complementary tests. In this section, we will only describe the diagnostic algorithm proposed in this ICD for indicating sleep studies.
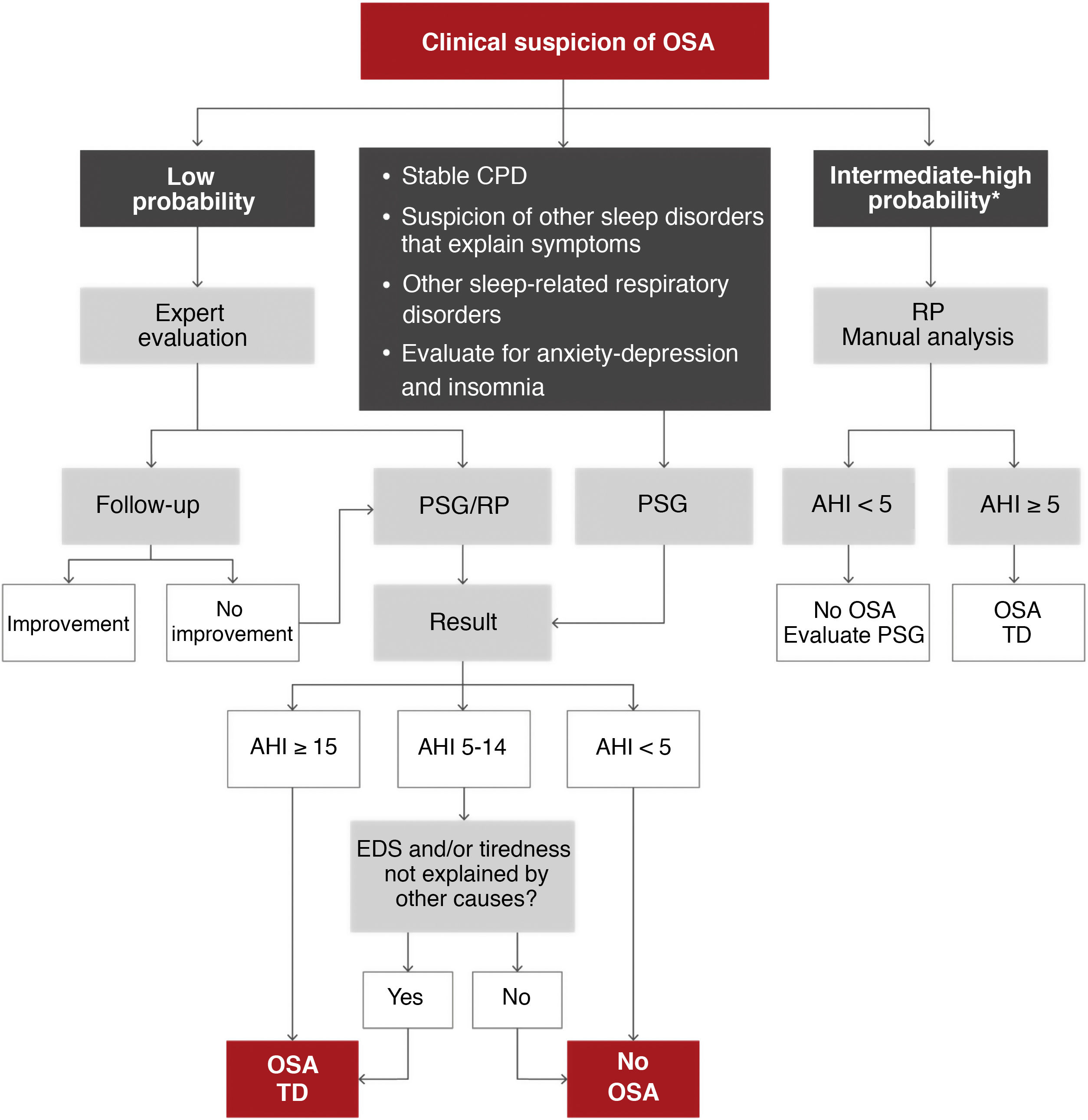
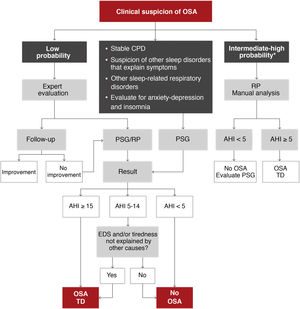
As OSA is a highly prevalent disease, it should be managed at different healthcare levels in order to satisfy the demand for care7. Two diagnostic algorithms are proposed. In specialized centers (Fig. 5), patients with moderate-to-severe chronic respiratory disease, unstable cardiovascular disease, other suspected sleep disorders that can cause symptoms or coexist with OSA and some patients with anxiety-depression or insomnia may be candidates for polysomnography (PSG) studies. Patients with a low probability of disease, according to an expert evaluation, may be candidates for follow-up and correction of other influencing factors, or a decision may be taken to perform PSG or respiratory polygraphy. Patients with an intermediate to high probability of OSA can be evaluated by respiratory polygraphy.
Proposed algorithm for the diagnosis of patients with suspected obstructive sleep apnea (OSA) at a specialized level. AHI: apnea-hypopnea index; CPD: cardiopulmonary disease; EDS: excessive daytime sleepiness; PSG: polysomnography; RP: respiratory polygraphy; TD: therapeutic decision.
*Intermediate-high probability is defined as the presence of EDS (Epworth > 10) and 2 of the following 3 criteria: usual intense snoring, observed choking arousals or apneas, and/or arterial hypertension.
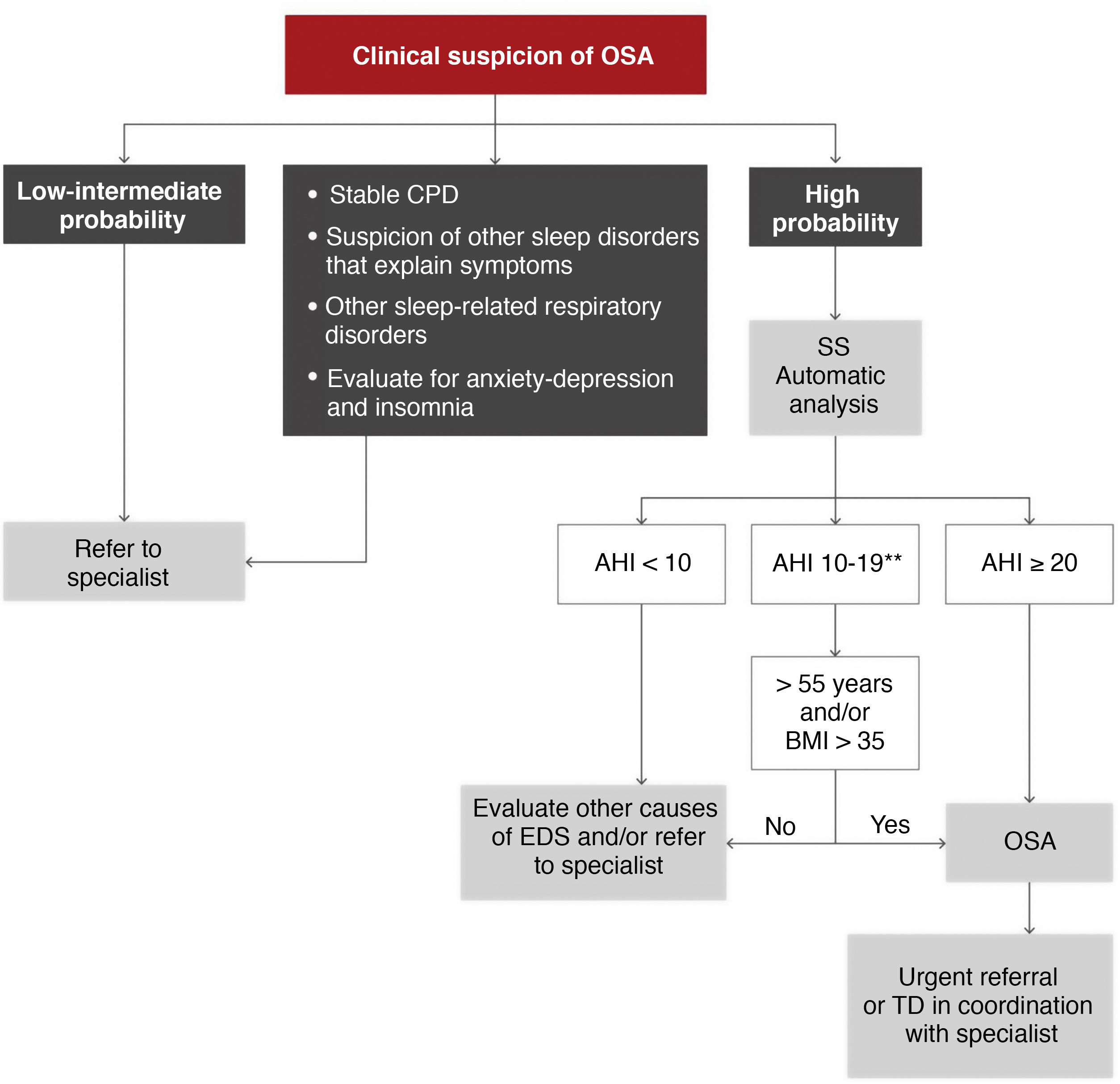
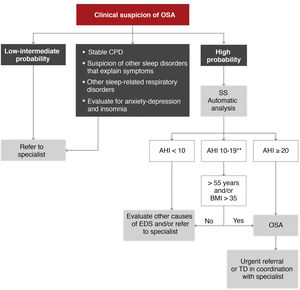
A second algorithm is proposed for primary care (Fig. 6), in which patients with a high probability of disease due to excessive daytime symptoms (Epworth ≥ 12) could be evaluated by simplified studies with single- or double-channel devices based on oximetry and/or nasal pressure8. It should be emphasized that this management must always be conducted in coordination with a reference sleep laboratory that can offer the necessary support, using protocols adjusted to specific needs. A therapeutic decision could be made in primary care and coordinated by specialists, but these models may be less generalizable, and they would have to be used in specific, previously validated areas9.
Proposed diagnostic algorithm in patients with suspected obstructive sleep apnea (OSA) seen in primary care and coordinated with the reference sleep laboratory. AHI: apnea-hypopnea index; BMI: body mass index; CPD: cardiopulmonary disease; EDS: excessive daytime sleepiness; SS: simplified study (one or 2 channels corresponding to nasal pressure and oximetry); TD: therapeutic decision.
*High probability is defined as the presence of EDS (Epworth ≥ 12) and 2 of the following 3 criteria: habitual intense snoring, observed choking arousals or apneas, and/or arterial hypertension.
**In these cases, a manual analysis of the recording by the coordinating sleep laboratory may offer a more accurate diagnosis.
The goals of OSA treatment are to resolve the signs and symptoms of the disease, restore sleep quality, normalize AHI, improve oxygen saturation as far as possible, reduce the risk of complications, and lower the costs of the disease. This ICD emphasizes that the various alternatives are combinable and recommends a multidisciplinary therapeutic approach.
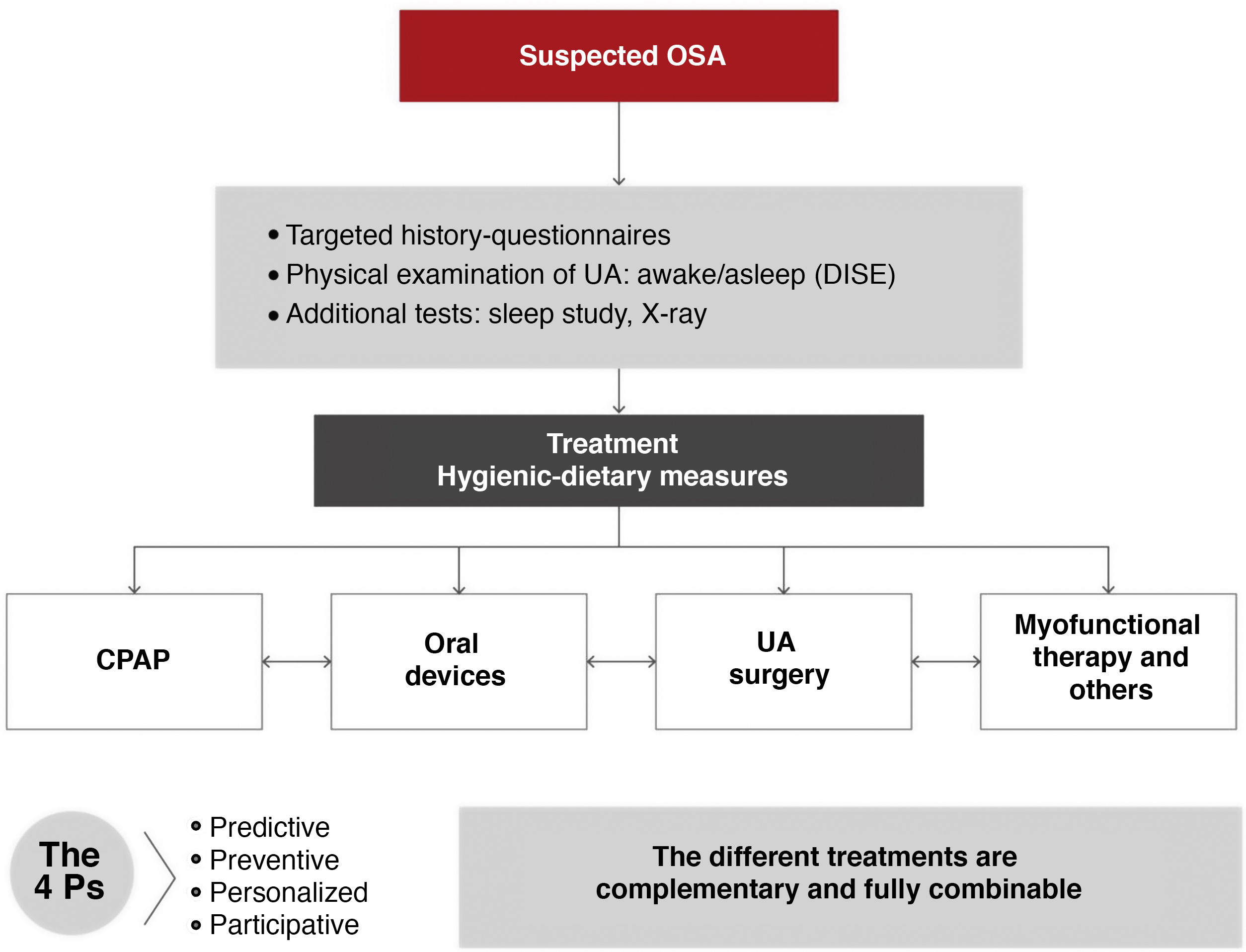
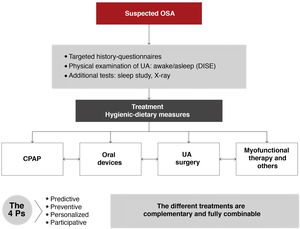
All medical, surgical, and physical options for the treatment of OSA should be complementary, not exclusive. Each patient should be offered the widest range of possibilities, and all strategies should be applied rationally, either alone or in combination, and individually adapted after an in-depth study. The patient's role in therapeutic decision-making must be emphasized. This should be the standard approach in multidisciplinary teamwork1–3,6,8 (Fig. 7).
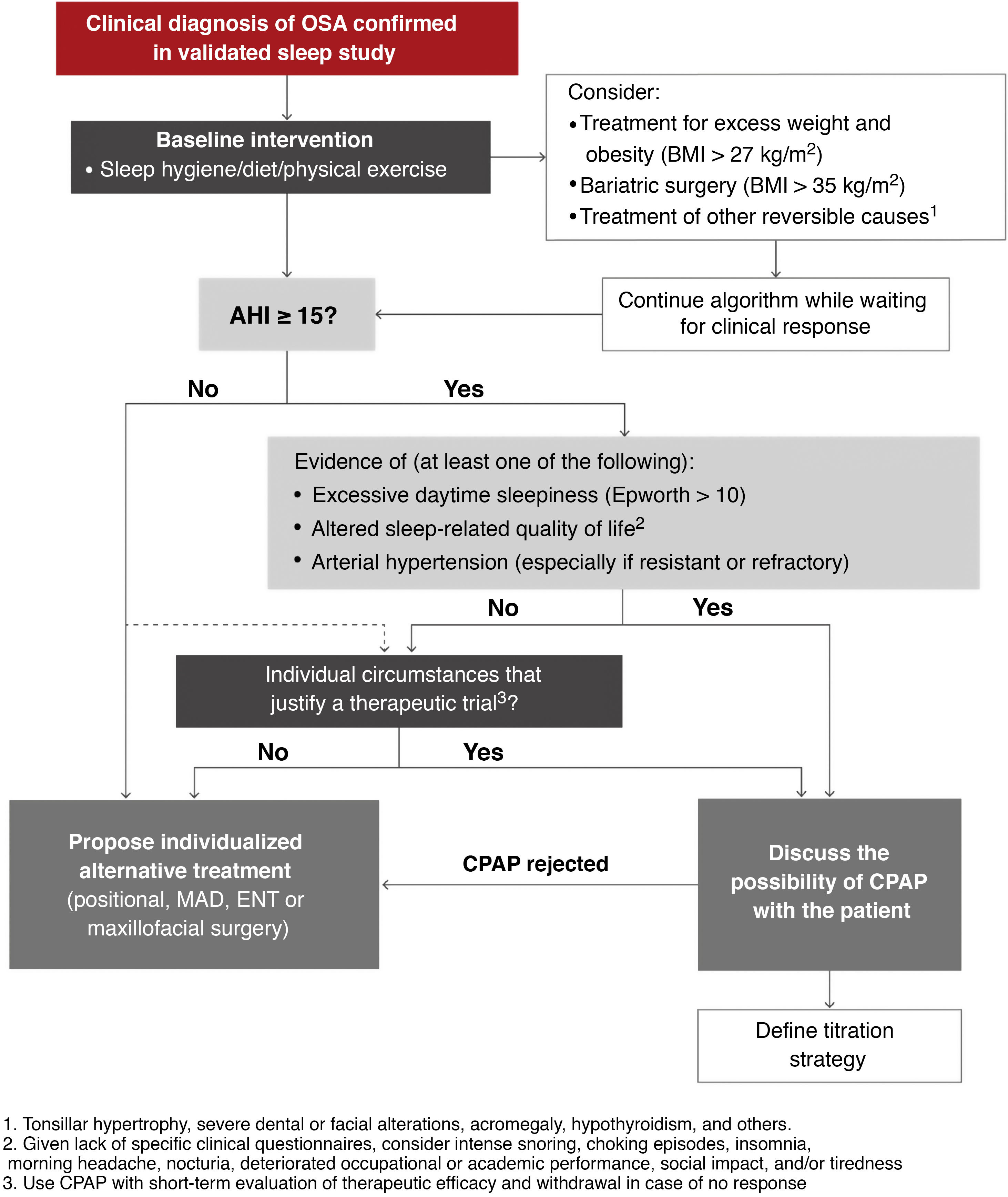
It is important to note that before starting any of the therapeutic alternatives, the clinical diagnosis of OSA must be confirmed by a sleep study validated according to the previously recommended diagnostic algorithm. The therapeutic algorithm (Fig. 8) is as follows:
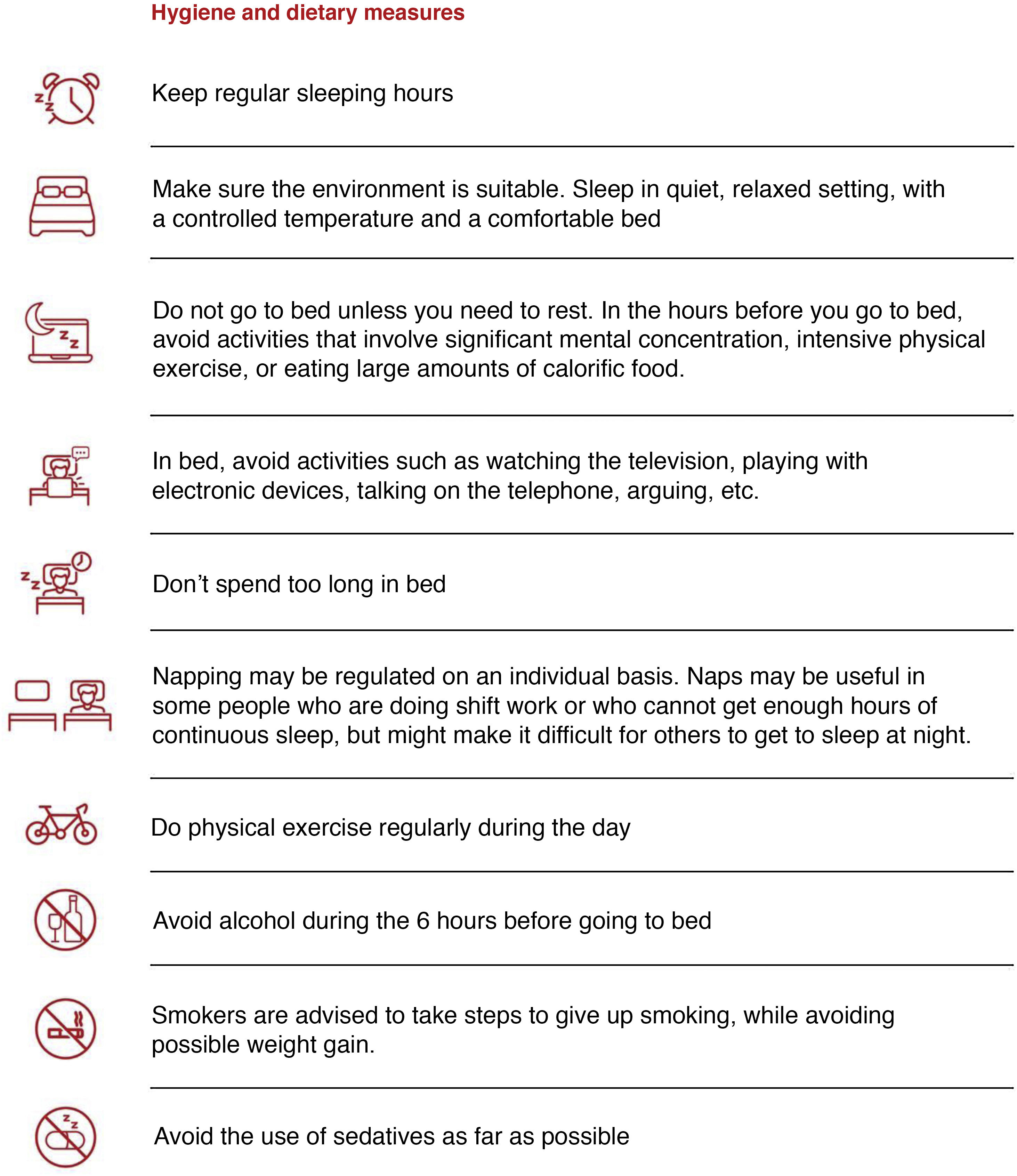
- 1
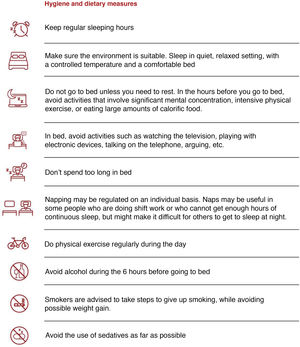
Hygienic-dietary measures should be implemented (Fig. 9) in all patients with OSA, regardless of whether continuous positive airway pressure (CPAP) therapy is indicated.
- 2
The patient must be evaluated to identify conditions associated with OSA and potentially reversible causes. Accordingly, this ICD recommends:
- •
Treatment of obesity: excess weight or obesity should be treated in all patients with OSA. Initial treatment should be part of a comprehensive, high-intensity program that includes behavioral strategies10. Severe obesity requires more durable strategies that should be evaluated in specialized units, where the use of anti-obesity drugs10 or surgery (for patients > 35 kg/m2) will be evaluated when conservative treatment fails11–14.
- •
Treatment of reversible causes: thyroid hormone replacement therapy is recommended in patients with OSA and hypothyroidism, so levels should be determined in case of clinical suspicion of hypothyroidism15. In case of gastroesophageal reflux, positional and dietary measures should be indicated, and treatment with proton pump inhibitors should be offered on an individual basis. If the patient presents tonsillar hypertrophy grade III/IV or severe dental or facial alterations, surgery to treat OSA should be considered.
- •
Proposed therapeutic algorithm for obstructive sleep apnea (OSA). A more detailed description of the scientific evidence that supports this algorithm can be found in the online material. AHI: apnea-hypopnea index; BMI: body mass index; CPAP: continuous positive airway pressure; ENT: ear, nose, and throat; MAD: mandibular advancement devices.
In any of these situations, the need for CPAP until treatment of the reversible cause becomes effective can be assessed.
- 3
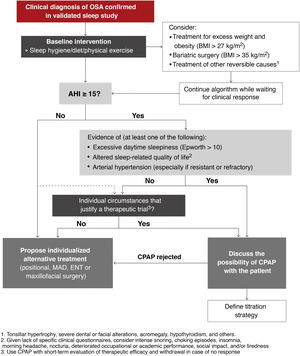
Indications for CPAP: CPAP is an effective treatment to reduce OSA severity, assessed according to AHI, and remains the treatment of choice in many of these patients16–29. Once the steps described above have been completed, the following recommendations for indicating CPAP, based on quality evidence evaluated according to currently available information in line with American Academy of Sleep Medicine guidelines30,31, should be followed.
A detailed explanation of the scientific evidence supporting selected cut-off points, symptoms and/or comorbidities used to determine the indication of CPAP can be found in the online material. This ICD recommends CPAP in:
- •
Patients with a targeted diagnosis of moderate-severe OSA (AHI ≥ 15/h) with excessive daytime sleepiness (Epworth > 10), altered sleep-related quality of life (intense snoring, episodes of night choking, insomnia, morning headache, nocturia, impaired work or academic performance, social impact, and/or tiredness during the day) and/or HBP (especially if resistant or refractory).
- •
In patients with no indication for CPAP due to AHI ≥ 15/h who do not present the above criteria, AHI between 5 and 15/h, or in whom CPAP is indicated but rejected (refusal to accept treatment or treatment unsuccessfully attempted for less than 4 weeks), alternative treatments should be assessed individually (mandibular advancement devices [MAD], positional treatment, surgery, etc.). These treatments and their indications are described in detail in the online material of this ICD.
- •
Insufficient evidence is available to consistently recommend the use of CPAP to reduce the risk of death or cardiovascular or cerebrovascular events in adults who do not meet the 3 criteria listed above. These patients should be offered conservative treatment with monitoring of symptoms or an individualized assessment including a CPAP trial (with short-term reassessment of treatment continuity depending on efficacy and tolerance).
- •
Similarly, in patients with OSA who have AHI < 15/h but are highly symptomatic or have a high burden of cardiovascular, cerebrovascular, or metabolic disease, a CPAP trial may be considered if the patient agrees. Current evidence suggests that CPAP may play a greater role in preventing cerebrovascular events than cardiovascular events32.
- •
Alternative treatments should be considered individually if the therapeutic trial fails.
The scientific evidence supporting these recommendations and a more detailed description can be found in the online material.
Adequate pressure titration and monitoring of CPAP compliance are essential to achieve the treatment objectives described. Please refer to the online material for a detailed description of these factors. In short, this ICD recommends considering PSG pressure titration for patients with significant (severe COPD) or unstable cardiopulmonary disease (heart failure), complex sleep-disordered breathing (central sleep apnea, suspected incipient central sleep apnea, or obesity-hypoventilation syndrome), or when titration with simplified methods has not been possible. For all other patients, pressure titration with auto-CPAP provides a level of OSA control similar to PSG titration. It is essential that the patient be trained before titration is attempted. This document recommends visual analysis of the graph and selection of the minimum pressure that, regardless of leakage peaks, covers about 90% of the entire pressure graph. It is also recommended that at least 5 valid hours of recording be examined. Finally, empirical formula calculation should only be considered between the start of treatment and until the definitive titration study (auto-CPAP, CPAP with memory card, or manual titration) is performed.
Compliance during the first 3 months can predict long-term use of the device33, so careful attention in this period will be key to achieving adequate long-term compliance34. This ICD defines good adherence as the use of the device for at least 4 h/night on 70% of nights. The current evidence points to a dose-response relationship between hours of use and therapeutic response35,36, and the neurocognitive and cardiovascular effects of CPAP and the perceived benefits in quality of life depend on this compliance37. For this reason, we propose the concept of optimal compliance with a minimum of 6 h/night, an approach which has shown benefits in symptom control and morbidity. As for the type of device, the use of auto-CPAP has not been shown to increase the percentage of nights with more than 4 h use38,39. Therefore, its use is only recommended for patients with high or highly variable effective pressure throughout the night.
Interventions for improving adherence are described in detail in the online material. Since the evidence suggests that telemonitoring improves CPAP adherence40,41, this strategy should be considered during the initial period of CPAP treatment. If it is used, remote recording of CPAP parameters30,31 should include hours of use, residual AHI, unintentional leaks, and machine configuration30,31,42.
Multidisciplinary management with the participation of a sleep specialist, the nursing team, and the suppliers will be important in the follow-up of patients receiving CPAP. Primary care should be included in long-term monitoring.
All patients receiving CPAP should be monitored after the first month of treatment and a brief in-person review should be performed at 6 months. Adherence at 3 months can be determined from telemonitoring. If the treatment is well established, with good clinical response and no side effects, the patient may be referred to primary care for follow-up after the first year.
If the patient has frank and proven intolerance to CPAP (the patient has tried to use it for more than 4 weeks, but has not been able to adapt), withdrawal should be considered. If either rejection or intolerance cannot be rectified, other therapeutic alternatives should be considered. In the event of clear lack of adherence with an average use of less than 3 h/night, the patient should be included in a compliance program, preferably with a telemonitoring system, and re-evaluated after at least 3 months, before assessing the possible withdrawal of CPAP and an alternative therapeutic proposal. Occasionally, patients who use CPAP for less than 3 h/night report symptomatic improvements, so the decision to discontinue treatment should be individualized.
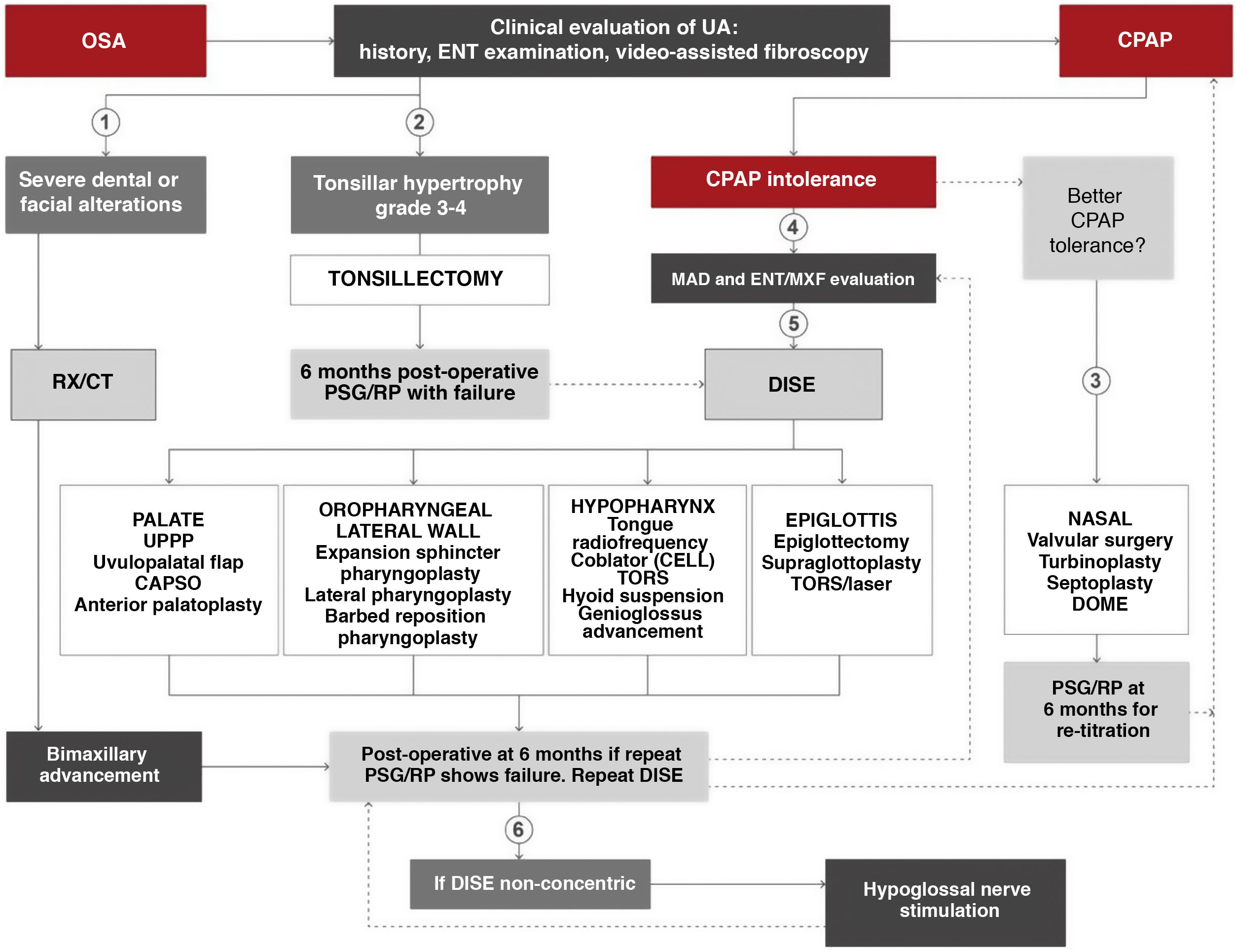
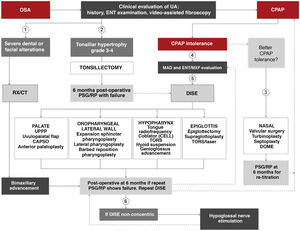
Surgical treatmentThe surgical algorithm recommended in this ICD for use by ENT specialists and oral or maxillofacial surgeons is an update of the standard Stanford 2-tiered protocol (Fig. 10)43,44. In this era of personalized medicine, a precise indication for palatal and oropharyngeal surgeries may initially be made based on clinical findings and drug-induced sedation endoscopy (DISE)45–47, but skeletal surgery, especially bimaxillary advancement, may also be indicated as initial surgical treatment of OSA in patients with severe disease (AHI > 65 and/or concentric collapse on DISE and/or severe dental and facial alterations). An indication for surgery never definitively excludes other treatments, or vice versa. Refer to the online material for a more detailed description of the systematic review and indications for surgery in OSA patients.
Algorithm for indicating surgical treatment in obstructive sleep apnea (OSA) using personalized precision medicine. CAPSO: cautery-assisted palatal stiffening operation; CELL: coblator endoscopic lingual lightening; CPAP: continuous positive airway pressure; DISE: drug-induced sleep endoscopy; DOME: distraction osteogenesis maxillary expansion; ENT/MXF; ear, nose and throat/maxillofacial; MAD: mandibular advancement device; PSG: polysomnography; RP: respiratory polygraph; RX/CT: radiological studies; TORS: transoral robotic surgery; UA: upper airway; UPPP: uvulopalatopharyngoplasty. (1) In case of severe dental or facial alterations, bimaxillary advancement may be indicated directly, although CPAP will be prescribed until surgery is conducted. (2) In case of tonsillar hypertrophy grade ≥ 3, tonsillectomy may be indicated directly, although CPAP will be prescribed until surgery. (3) In case of CPAP intolerance after ORL evaluation, nasal surgery is recommended as soon as possible to improve intolerance and retitrate CPAP. (4) In case of CPAP intolerance after dental/maxillofacial evaluation, adaptation of MAD or assessment for orthognathic surgery is recommended. (5) In case of CPAP intolerance after ENT evaluation, DISE is recommended to determine the most appropriate upper airway treatment. (6) After intolerance to CPAP and failure of other surgeries, a repeat DISE is recommended. If collapse is non-concentric, hypoglossal neurostimulation may be indicated.
The proposed algorithm moves on from the classic concept of soft tissue removal or skeletal modification44. It divides the surgical procedures to be performed according to the affected organ, and the choice of procedure is based primarily on the exploratory and diagnostic findings and on the final decision of the patient after all the options have been explained, none of which is either exclusive or prevailing. It is common for patients to have airway obstructions at different levels, so the current tendency is to perform multilevel surgery, in which, once the different sites of upper airway obstruction have been determined, a decision is made on the different procedures to be performed, alone or in combination, aiming to achieve optimal results48–51.
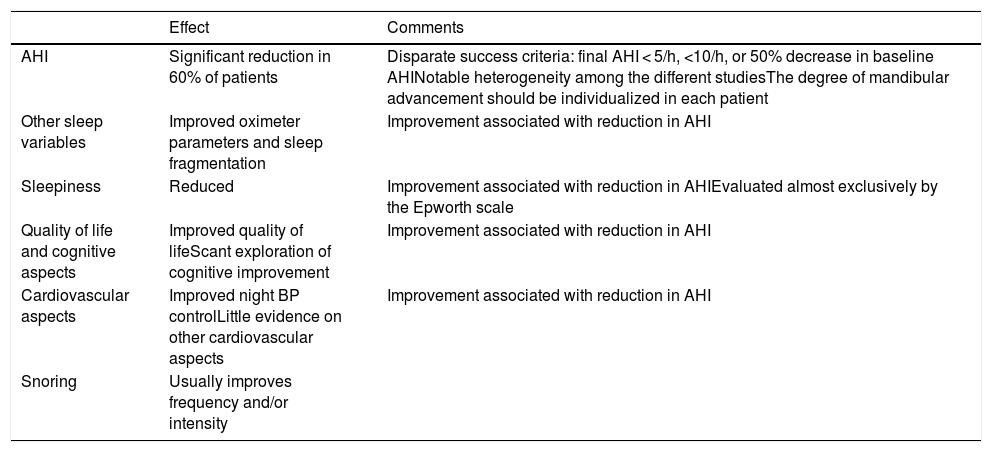
Treatment with mandibular advancement devicesRecent randomized controlled trials have demonstrated the efficacy of MADs on various aspects of OSA as summarized in Table 4 (see also online material):
Summary of existing scientific evidence on the efficacy of mandibular advancement device in patients with obstructive sleep apnea.
| Effect | Comments | |
|---|---|---|
| AHI | Significant reduction in 60% of patients | Disparate success criteria: final AHI < 5/h, <10/h, or 50% decrease in baseline AHINotable heterogeneity among the different studiesThe degree of mandibular advancement should be individualized in each patient |
| Other sleep variables | Improved oximeter parameters and sleep fragmentation | Improvement associated with reduction in AHI |
| Sleepiness | Reduced | Improvement associated with reduction in AHIEvaluated almost exclusively by the Epworth scale |
| Quality of life and cognitive aspects | Improved quality of lifeScant exploration of cognitive improvement | Improvement associated with reduction in AHI |
| Cardiovascular aspects | Improved night BP controlLittle evidence on other cardiovascular aspects | Improvement associated with reduction in AHI |
| Snoring | Usually improves frequency and/or intensity |
AHI: apnea-hypopnea index.
This ICD therefore recommends that52,53:
- •
The diagnosis of OSA and the efficacy of MAD should always be determined by respiratory polygraphy or PSG.
- •
The indications for MAD are:
- 1
Patients with OSA of any severity who are candidates for CPAP therapy but unable to adapt to it. MAD is principally indicated as an alternative to CPAP and should be available in sleep units in the public health system, or
- 2
Patients with mild to moderate OSA, minor symptoms, or troublesome snoring and no indication for CPAP or any other treatment.
- 1
- •
Before prescribing an MAD, the dentist must perform an oral examination to rule out patients who do not meet dental inclusion criteria.
- •
Current evidence supports the use of custom-made, adjustable devices.
- •
Following assessment of the patient's suitability from the point of view of oral health, treatment should be implemented and followed up by a certified dentist or OSA sleep-disordered breathing expert working in coordination with a sleep unit. Respiratory polygraphy may be used by an experienced sleep dentist as a tool for MAD titration.
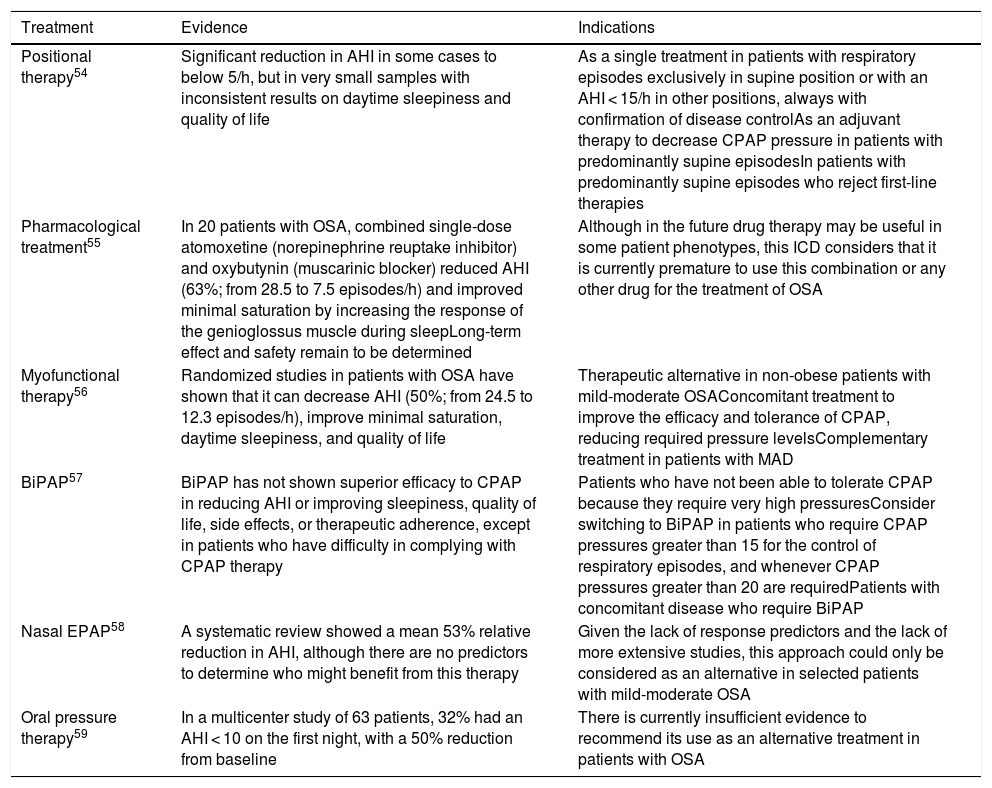
OSA is a heterogeneous disease in terms of both its pathophysiology and its polysomnographic expression and clinical presentation. In recent years, various OSA phenotypes have been described that are explained in depth in the online material of this document. This phenotype approach has helped improve our knowledge of the mechanisms involved in the disease and the development of different therapeutic strategies, and has contributed to the development of a more personalized medicine. Table 5 summarizes current evidence and indications for other alternative therapies (see also online material).
Summary of current evidence and indications for alternative therapies in the management of OSA.
| Treatment | Evidence | Indications |
|---|---|---|
| Positional therapy54 | Significant reduction in AHI in some cases to below 5/h, but in very small samples with inconsistent results on daytime sleepiness and quality of life | As a single treatment in patients with respiratory episodes exclusively in supine position or with an AHI < 15/h in other positions, always with confirmation of disease controlAs an adjuvant therapy to decrease CPAP pressure in patients with predominantly supine episodesIn patients with predominantly supine episodes who reject first-line therapies |
| Pharmacological treatment55 | In 20 patients with OSA, combined single-dose atomoxetine (norepinephrine reuptake inhibitor) and oxybutynin (muscarinic blocker) reduced AHI (63%; from 28.5 to 7.5 episodes/h) and improved minimal saturation by increasing the response of the genioglossus muscle during sleepLong-term effect and safety remain to be determined | Although in the future drug therapy may be useful in some patient phenotypes, this ICD considers that it is currently premature to use this combination or any other drug for the treatment of OSA |
| Myofunctional therapy56 | Randomized studies in patients with OSA have shown that it can decrease AHI (50%; from 24.5 to 12.3 episodes/h), improve minimal saturation, daytime sleepiness, and quality of life | Therapeutic alternative in non-obese patients with mild-moderate OSAConcomitant treatment to improve the efficacy and tolerance of CPAP, reducing required pressure levelsComplementary treatment in patients with MAD |
| BiPAP57 | BiPAP has not shown superior efficacy to CPAP in reducing AHI or improving sleepiness, quality of life, side effects, or therapeutic adherence, except in patients who have difficulty in complying with CPAP therapy | Patients who have not been able to tolerate CPAP because they require very high pressuresConsider switching to BiPAP in patients who require CPAP pressures greater than 15 for the control of respiratory episodes, and whenever CPAP pressures greater than 20 are requiredPatients with concomitant disease who require BiPAP |
| Nasal EPAP58 | A systematic review showed a mean 53% relative reduction in AHI, although there are no predictors to determine who might benefit from this therapy | Given the lack of response predictors and the lack of more extensive studies, this approach could only be considered as an alternative in selected patients with mild-moderate OSA |
| Oral pressure therapy59 | In a multicenter study of 63 patients, 32% had an AHI < 10 on the first night, with a 50% reduction from baseline | There is currently insufficient evidence to recommend its use as an alternative treatment in patients with OSA |
AHI: apnea-hypopnea index; BiPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; EPAP: expiratory positive airway pressure; ICD: international consensus document; MAD: mandibular advancement devices; OSA: obstructive sleep apnea.
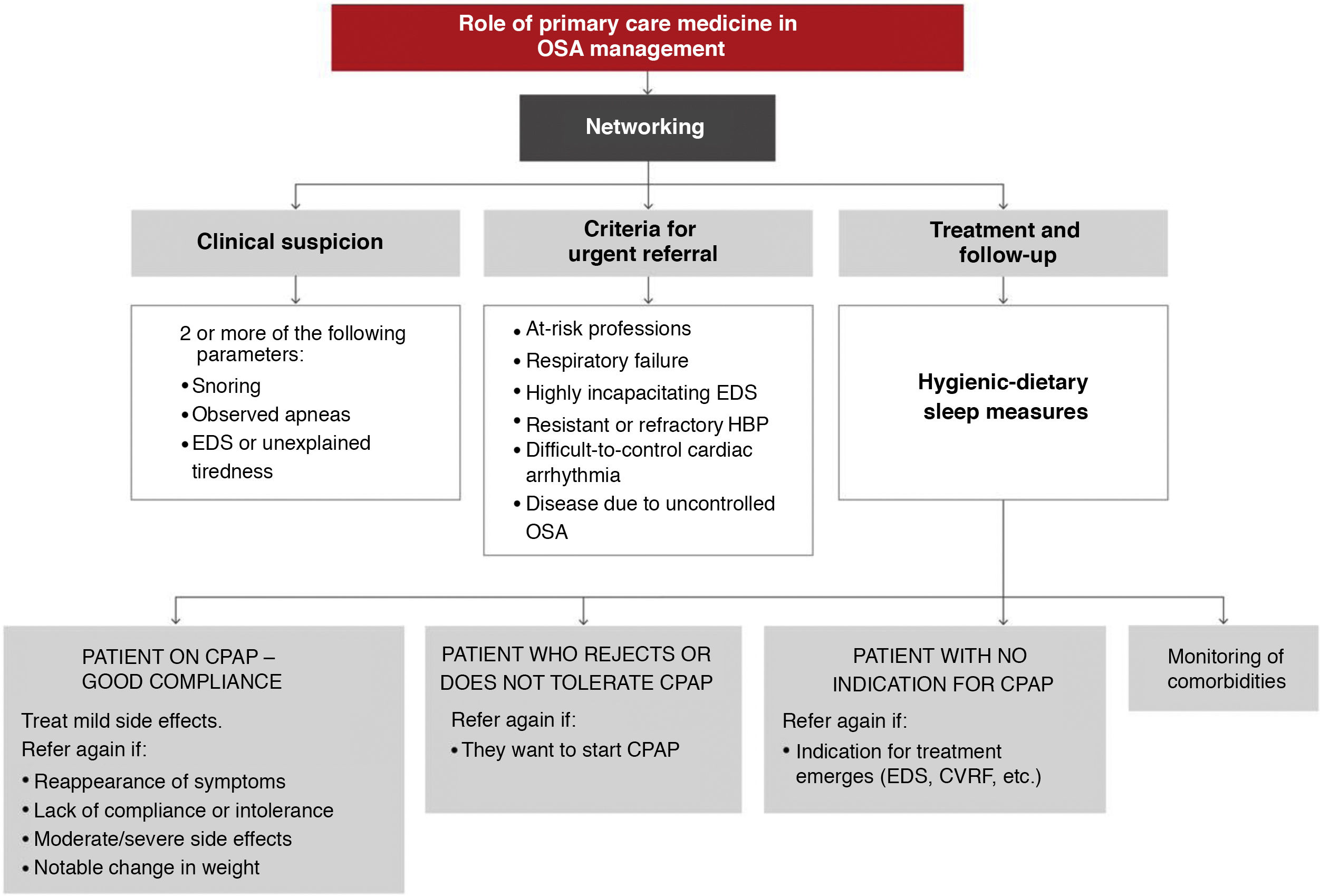
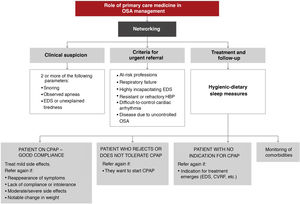
In light of the current evidence, the recommendations of this ICD on the role of primary care in the management of OSA patients are as follows:
- 1
The involvement of primary care physicians is essential to improve the current situation of OSA underdiagnosis.
- 2
The implementation of training plans in primary care improves the suspicion and diagnostic process of OSA.
- 3
The criterion for clinical suspicion should be the presence of 2 of the 3 main symptoms: snoring, observed apneas, and/or excessive daytime sleepiness or unexplained intense tiredness.
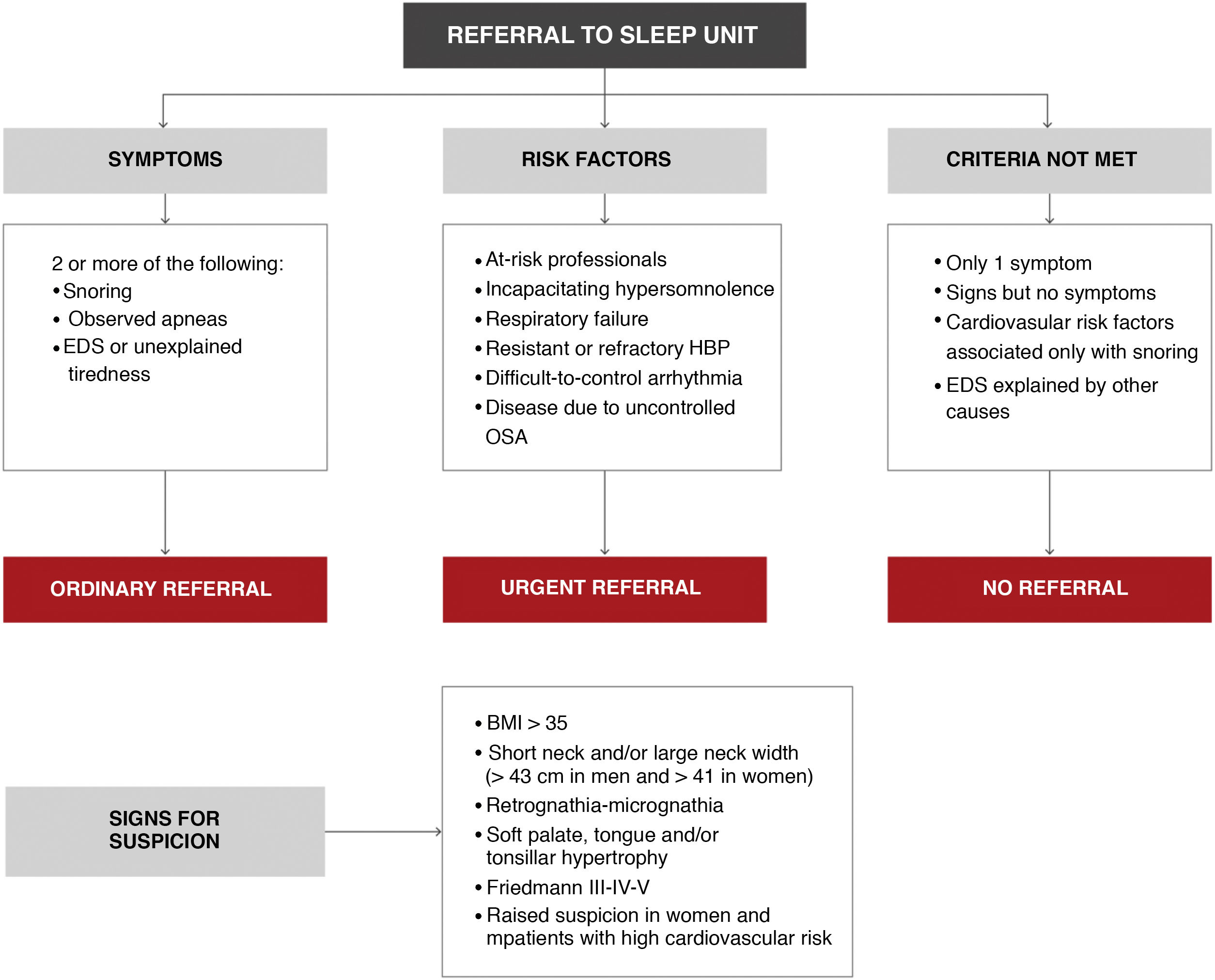
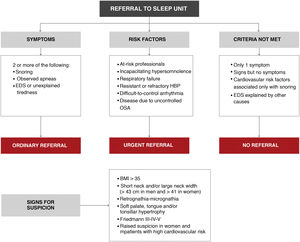
- 4
The situations that require urgent referral are summarized in Fig. 11:
- a)
Primary care diagnostic procedures should be performed in consensus with the reference sleep unit, working in a network.
- b)
At the moment, evidence on how to start CPAP treatment exclusively in a primary care setting is insufficient.
- c)
Most treatment follow-up requirements can be met in primary care.
- a)
These activities should be conducted as indicated in Fig. 12. A more detailed explanation can be found in the online material.
ConclusionsOSA is a highly prevalent disease with significant consequences. Its diagnostic and therapeutic management requires multidisciplinary treatment and involves all levels of care. Identification of possible reversible causes and assessment of all treatment options, all of which are combinable, will contribute to comprehensive patient management.
AuthorshipAll the authors have participated in the study and read and approved the manuscript.
Conflict of interestsPedro García Ramos states that he has collaborated with GSK, Laboratorios Ferrer, Angelini, Novartis, Almirall, Gebro Pharma, Rovi, Esteve, Recordati, MSD, and Teva. Carlos Teixeira states that he is an employee of Philips (Sleep and Respiratory Care Division). Francisco Javier Puertas Cuesta states that he has received fees for consultancy and speaking in courses and seminars from Jazz Pharmaceuticals, UCB Pharma, GSK, Esteve Teijin, and ResMed, and has received research grants from Philips. The other authors state that they have no conflict of interests with the manuscript.
Esteve Teijin organized the first national in-person meeting. Philips (Sleep and Respiratory Care Division) organized the final in-person document review meeting. None of these companies was involved in the scientific discussion or the drafting of the document. Ivan Solà participated in the research work as an expert documentalist. This ICD is especially dedicated to our companion Mari Luz Alonso-Álvarez, who always set an example of best practices in the quest for scientific evidence.
The following are Supplementary data to this article:
Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), Spanish Society of Neurology (SEN), Spanish Society of General and Family Physicians (SEMG), Spanish Society of Dental Sleep Medicine (SEMDeS), Spanish Society of Clinical Neurophysiology (SENFC), Spanish Society of Endocrinology and Nutrition (SEEN), Spanish Society of Oral and Maxillofacial and Head and Neck Surgery (SECOM CYC), Spanish Society of Family and Community Medicine (semFYC), Spanish Society of Traffic Medicine (SEMT), Spanish Society of Otorhinolaryngology and Cervical Surgery (SEORL-CCC), Spanish Society of Cardiology (SEC), Spanish Society of Sleep (SES), Spanish Society for the Study of Obesity (SEEDO).
Latin American Thoracic Association (ALAT), Brazilian Society of Pulmonology and Phthisiology (SBPT), Portuguese Society of Pulmonology (SPP), SomnoNIV Group of the French Language Pulmonology Society (SPLF).
Please cite this article as: Mediano O, González Mangado N, Montserrat JM, Alonso-Álvarez ML, Almendros I, Alonso-Fernández A, et al., Documento internacional de consenso sobre apnea obstructiva del sueño. Arch Bronconeumol. 2022;58:52–68.