The use of ultrasound in peripheral thoracic lesions offers advantages over other radiological guiding methods. This diagnostic procedure has been applied in most studies published by radiologists. Our aim was to determine the diagnostic efficacy of percutaneous ultrasound-guided punctures and biopsies of peripheral thoracic lesions performed by pulmonologists.
MethodologyA retrospective analysis of 58 patients who underwent real-time ultrasound-guided transthoracic punctures and biopsy of peripheral thoracic lesions between March 2011 and September 2014 in the pulmonology department of our hospital, was carried out. Cases were classified into the following diagnostic categories: malignant, benign and non-diagnostic (non-specific benign without evidence of malignancy and insufficient specimen).
ResultsA conclusive diagnosis was obtained in 47 procedures (81%), of which 13 (22.4%) were specific benign lesions and 34 (58.6%) cancers. In the remaining 11 (19%) patients, a non-diagnostic result was obtained [non-specific benign in 5 cases (8.6%) and insufficient specimen in 6 (10.3%)]. Sensitivity was 75.6%, negative predictive value was 54.2%, specificity and positive predictive value were 100%, and diagnostic accuracy was 81%. Excluding procedures with insufficient specimens, the results were 87.2%, 72.3%, 100%, 100% and 90.4% respectively. There were no serious complications.
ConclusionsPercutaneous ultrasound-guided puncture and biopsy in the diagnosis of peripheral thoracic lesions performed by pulmonologists is a safe procedure with high diagnostic accuracy. We achieved similar results to those previously obtained by radiologists.
La ecografía como guía en la punción percutánea de lesiones torácicas periféricas (LTP) ofrece ventajas frente a otras técnicas de imagen. La mayoría de los estudios con esta técnica han sido comunicados por radiólogos intervencionistas. El objetivo de este estudio ha sido analizar la rentabilidad diagnóstica de la punción percutánea guiada por ecografía en una unidad de técnicas de neumología.
MetodologíaEstudio retrospectivo de 58 pacientes con LTP puncionadas con visualización ecográfica en tiempo real, entre el 1 de marzo de 2011 y el 1 de septiembre de 2014. Los resultados fueron divididos en 3 categorías diagnósticas: maligna, benigna y no diagnóstica (ND); esta última incluye los resultados de benignidad no específica (SD) y los de muestra insuficiente para diagnóstico (MID).
ResultadosSe obtuvo: resultado maligno en 34 (58,6%) de los procedimientos, resultado benigno en 13 (22,4%) y ND en 11 (19%) (SD en 5 [8,6%] y MID en 6 [10,3%]). En 5 de los casos ND el resultado final fue de malignidad y en 4 de ellos se tratada de una MID. La sensibilidad diagnóstica obtenida fue del 75,6%, el valor predictivo negativo del 54,2%, y la especificidad y el valor predictivo positivo del 100%, con una rentabilidad diagnóstica del 81%. Cuando se excluyeron los casos con MID los valores fueron del 87,2%, 72,3%, 100% y 100%, respectivamente, con una rentabilidad diagnóstica del 90,4%. No hubo complicaciones graves con la técnica.
ConclusionesLa punción percutánea bajo guía ecográfica en LTP realizada por neumólogos intervencionistas es una técnica segura y con una alta rentabilidad diagnóstica.
Chest ultrasound (US) is a safe and effective method of evaluating lesions in the chest wall, pleural cavity, mediastinum and the lung periphery.1,2 Using US to guide needle biopsy to obtain specimens for histocytology studies provides real-time imaging of the procedure.3 Performing needle biopsy under US guidance has many advantages over other imaging techniques: it does not expose the patient to radiation, the equipment is easily transported, and the procedure is quick, inexpensive, and can be performed at the bedside.4,5 US-guided techniques are particularly suitable for individuals that are more susceptible to injury from radiation, such as children and pregnant women, and for patients that are difficult to move, such as those admitted to intensive care units.6
Estimates suggest that 40% of pulmonary malignancies appear as masses in peripheral lung tissue, and are potentially accessible to US.7 Despite these advantages, US is rarely used in Spain in the study of malignant chest lesions, and in most hospitals the technique of choice is computed tomography (CT)-guided needle biopsy.4
In our unit, we have routinely used US since 2010 to detect pleural effusion and to guide thoracocentesis. In 2011, we started to use real-time US imaging to guide needle biopsy in pleural, pulmonary and mediastinal lesions.
Most studies involving this technique have been authored by interventional radiologists, and there is scant reference in the literature to the experience of pulmonologists in this context.2,8–10
A review of the literature showed no published series from Spanish hospitals.
The aim of this study was to evaluate the cost-effectiveness and safety of US-guided puncture and/or biopsy in the diagnosis of peripheral chest lesions in an interventional pulmonology unit (IPU).
Materials and MethodsPatient Selection and Data CollectionThis is a retrospective study of all patients undergoing US-guided needle biopsy with real-time imaging to diagnose peripheral thoracic lesions. The study was conducted from March 1, 2011 to September 1, 2014. Information on the procedure was obtained from records stored in the ENDOBASE® database (Olympus, Tokyo, Japan), and demographic, clinical, histopathological details, together with complications and patient outcomes, were obtained from electronic clinical records.
All patients, except 1, had undergone chest CT scan prior to the procedure. These images were used as a reference to determine the location and size of the thoracic lesion. In the patient with no previous CT scan, the size of the lesion was calculated using US imaging. Lesions were classified according to their maximum diameter as nodules (≤3cm) or masses (>3cm).
All patients were followed up clinically and radiologically for between 6 and 48 months. Serious complications associated with the procedure were defined as: pneumothorax, clinically relevant bleeding, need for transfusion, need for chest drainage, or emergency hospitalization.
The ProcedureAs a prerequisite for the procedure, platelet count had to be higher than 100000/μl and activated partial thromboplastin time had to be within reference limits.
The US examination and core biopsy or fine needle aspiration (FNA) were performed simultaneously by staff from the interventional unit of the pulmonology department using a General Electric LOGIQ P6 ultrasound system (Solingen, Germany).
Intrathoracic lesions were initially evaluated using a 4MHz convex transducer. If the mass or nodule invaded the chest wall, a linear 7MHz transducer was also used (Fig. 1). Patients were placed supine, prone or lateral decubitus, according to the position which gave greater US access with the best safety profile.
In 50 cases, puncture was performed using a 22G Chiba® biopsy needle (Gallini, Italy), and 16 and 18 gauge Acecut® (TSK, Japan), Trucut® (Biopsybell, Italy) or Surecut® (TSK, Japan) needles were used in 14, 8, and 5 needle biopsies, respectively.
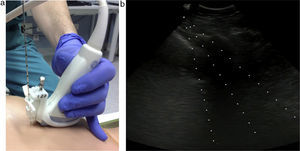
Both techniques (core biopsy and FNA) were guided by real-time US imaging. A needle guide was attached to the transducer (Fig. 2) to ensure precision.
The pathologist was not present during sample collection. All core and needle biopsies were performed under local anesthesia (mepivacaine 2%) of the skin and underlying tissue.
Classification of ResultsThe results of the cytology and/or biopsy were divided into 3 diagnostic categories: (1) malignant; (2) benign; and (3) non-diagnostic (ND). The first 2 categories (malignant and benign) were considered conclusive results. The ND category included cases where the diagnosis was inconclusive: non-specific benign without evidence of malignancy (NS) (non-specific cellularity/inflammatory cells or necrosis), and cases where the size of the sample was insufficient for diagnosis (IS).
Results were classified as malignant when a diagnosis of malignant neoplasm was obtained, and as benign when a specific diagnosis of benign lesion was obtained and the clinical picture was consistent with the pathological diagnosis.
Patients in whom the specimen obtained was non-diagnostic (ND) underwent alternative diagnostic procedures: new cytohistologic biopsy or clinical and radiological follow-up.
New specimens were collected by various means: by interventional radiologists (CT-guided or US-guided FNA); by pulmonologists using thoracocentesis or bronchoscopy with transbronchial biopsy; or by resection performed by thoracic surgeons.
Statistical AnalysisIf the analysis of the specimen showed the presence of neoplastic cells, the result was considered a true positive (TP), and no further confirmatory tests were performed.
If histology of the specimen (cytology and/or biopsy) was benign, the result was considered a true negative (TN) for malignancy following histology confirmation and/or evidence on CT or positron emission tomography (PET) scan after follow-up of between 6 and 48 months that the tumor had shrunk, was stable, or had disappeared despite receiving no specific antitumor treatment.
Benign histology was considered a false negative when a new specimen was ultimately positive for malignancy, or clinical and radiological follow-up indicated a malignant tumor (on CT or PET scan the tumor was shown to have grown or metastases were identified).
ND cases were not included in the analysis, as insufficient samples were available for an accurate malignant or benign diagnosis.
Quantitative variables are shown as mean (±standard deviation), and qualitative or dichotomous variables are shown as absolute percentages and frequencies.
Diagnostic yield was evaluated using a 2×2 contingency table, in which positive or negative results for malignancy were assigned to the rows and presence or absence of definitive diagnosis was assigned to the columns. This method gave the sensitivity (S), specificity (SP), positive predictive value (PPV), negative predictive value (NPV) and diagnostic yield of the test. S and NPV were determined after first considering all specimens, including ISs, and then selecting only specimens suitable for cytology study (excluding ISs).11
Diagnoses (procedures with conclusive and inconclusive results) were compared with different categorical variables using the χ2 test. Diagnoses were compared with the following categorical variables: size (nodule and mass) and method of collection (core biopsy and FNA). Diagnosis of histological subtypes in non-small cell lung cancer (NSCLC) was compared with the sample collection method using the χ2 test.
In all cases, significance was set at P<.05.
Statistical analysis was performed using the Statistical Package for Social Sciences, version 20.0 (SPSS, Chicago, IL, USA).
ResultsIn 15 (26%) cases, the lesion measured ≤3cm (nodule); in 43 (74%) cases, it measured >3cm.
In 51 (88%) cases, the lesion was located in the lung, in the mediastinum in 3 (5%) cases, in the parietal pleura in 2 (3.5%) cases, and supraclavicular region in 2 (3.5%) cases (Table 1).
Anthropometry, Location and Size of the Lesion, Type of Needle Used and Biopsy Procedure.
| Characteristics | Patients |
|---|---|
| Age, years (x±SD) | 60.86±14.14 |
| Sex (male/female) | 45/13 |
| Location (n, %) | |
| Right upper lobe | 18 (31.0) |
| Middle lobe | 2 (3.4) |
| Right lower lobe | 10 (17.2) |
| Left upper lobe | 16 (27.6) |
| Left lower lobe | 5 (8.6) |
| Anterior mediastinum | 3 (5.2) |
| Supraclavicular | 2 (3.4) |
| Parietal pleura | 2 (3.4) |
| Size of lesion (n, %) | |
| Nodule (≤3cm) | 15 (25.9) |
| Mass (>3cm) | 43 (74.1) |
| Type of needle | |
| Chiba® | 50 |
| Surecut® | 5 |
| Acecut® | 14 |
| Trucut® | 8 |
| No data available | 3 |
| Biopsy procedure (n, %) | |
| Only FNA | 28 (48.3) |
| Only core biopsy | 5 (8.6) |
| FNA+core biopsy | 22 (37.9) |
| No data available | 3 (5.2) |
FNA: fine needle aspiration.
Table 2 shows that a conclusive diagnosis (for benign or malignant disease) was obtained in 47 out of 58 (81%) procedures. The most frequent diagnosis was NSCLC (38%). Of the 11 (19%) non-diagnostic cases (Table 3), definitive diagnosis was obtained with CT-guided FNA performed by interventional radiologists (1 case), US-guided FNA performed by interventional radiologists (2 cases), thoracotomy (1 case), bronchoscopy with transbronchial biopsy (1 case), bronchoscopy with transbronchial biopsy (1 case), or on the basis of clinical and radiological follow-up (5 cases). Five cases initially classified as non-diagnostic for malignancy (benign and non-diagnostic) were later definitively diagnosed as malignant by the end of the study.
Study Patients by Diagnosis.
| Final Diagnosis | Number | % |
|---|---|---|
| Malignant | 34 | 58.6 |
| Non-small cell | 22 | 37.9 |
| Adenocarcinoma | 15/22 | |
| Squamous carcinoma | 5/22 | |
| Non-small cella | 2/22 | |
| Small cell | 4 | 6.9 |
| Metastasis | 2 | 3.4 |
| Other cancers | 6 | 10.3 |
| Benign | 13 | 22.4 |
| Tuberculosis | 5 | 8.6 |
| Abscess | 4 | 6.9 |
| Pachypleuritis/fibrothorax | 2 | 3.4 |
| Pleural fibroma | 1 | 1.7 |
| Granulomatous lymphadenitis | 1 | 1.7 |
| Non-diagnostic | 5 | 8.6 |
| Insufficient sample | 6 | 10.3 |
| Total | 58 | 100.00 |
Analysis of the Subgroup of Patients with Non-diagnostic Cytohistology or Insufficient Sample.
| Patient | Diagnosis | Size Measured by CT (cm) | Location | Type of Sample | Final Diagnostic Method | Final Diagnosis |
|---|---|---|---|---|---|---|
| #4 | Insufficient sample | 4 | LUL | FNA | Clinical and radiological follow-up | Pulmonary abscess |
| #5 | Insufficient sample | 8 | RLL | FNA | Clinical and radiological follow-up | Malignant neoplasm (patient declined further diagnostic tests) |
| #10 | Insufficient sample | 2.5 | SC | Biopsy | Bronchoscopy with TBB | Small cell carcinoma |
| #12 | Insufficient sample | 7 | LUL | FNA | US-guided FNA performed by interventional radiologist | Squamous cell lung cancer |
| #37 | Inflammatory cells (NS) | 1.9 | RLL | FNA+core biopsy | Negative CT-guided FNA -Benign nodule: radiological follow-up | Benign nodule |
| #39 | Inflammatory cells (NS) | 8.4 | PP | FNA+core biopsy | Positive thoracocentesis Clinical and radiological follow-up | Purulent mediastinitis |
| #41 | Necrosis (NS) | 9.6 | RUL | FNA+core biopsy | US-guided FNA performed by interventional radiologist (2 procedures on different days were required for a positive diagnosis) | Pulmonary sarcoma |
| #45 | Necrosis (NS) | 5.9 | AM | Core biopsy | Thoracotomy | Pulmonary echinococcosis |
| #49 | Insufficient sample | 3 | RUL | FNA | PET/CT follow-up | Scar tissue |
| #53 | Insufficient sample | 5 | AM | FNA | CT-guided FNA | B-cell lymphoma |
| #57 | Inflammatory cells (NS) | 6.3 | RUL | FNA+core biopsy | BAL positive for klebsiella Clinical and radiological follow-up | Klebsiella pneumonia |
AM: anterior mediastinum; BAL: bronchoalveolar lavage; FN: false negative, FNA: fine needle aspiration; LUL: left upper lobe; NS: non-specific; PET/CT: positron emission tomography/computer tomography; PP: parietal pleura; RLL: right lower lobe; RUL: right upper lobe; SC: supraclavicular; TBB: transbronchial biopsy; TN: true negative.
Table 4 shows the diagnostic yield of US-guided needle biopsy in the best (excluding unsuitable specimens) and worse clinical scenario (including unsuitable specimens and specimens with inflammatory cells).
Diagnostic Yield of Ultrasound-guided Needle Biopsy Including or Excluding Insufficient Samples.
| Variable | Including Insufficient Samples | Excluding Insufficient Samples |
|---|---|---|
| Number (n) | 58 | 52 |
| Non-diagnostic | 11 | 5 |
| Sensitivity | 75.6% | 87.2% |
| Specificity | 100% | 100% |
| NPV | 54.2% | 72.3% |
| Diagnostic yield | 81% | 90.4% |
NVP: negative predictive value.
Lesion size (nodule/mass) did not affect the process of obtaining a definitive diagnosis (P=.91).
No statistically significant differences were found between the specimen collection method (FNA, core biopsy, or FNA+core biopsy) and diagnostic yield (P=.96). Neither were any statistically significant differences found between diagnostic yield from specimens obtained with biopsy (core biopsy alone or with FNA) and those obtained with FNA alone (P=.75). When determining the histological subtype of the 22 patients diagnosed with NSCLC, excluding 2 patients in whom the type of needle used was unknown, no statistically significant differences were found between specimens obtained using FNA and core biopsy (with or without FNA).
No serious complications were associated with any of the biopsy procedures. One patient died shortly after the biopsy due to progression of malignant disease.
DiscussionThis study shows that US-guided needle biopsy performed by an interventional pulmonologist can be used to obtain a specific diagnosis (benign or malignant disease) of peripheral thoracic lesions in contact with the pleura in up to 81% of cases. Diagnostic yield of malignancy is high when sufficient fluid or tissue is obtained for diagnosis.
Our findings also show that US-guided biopsy can be used to establish a diagnosis of benign disease, such as infectious processes, particularly tuberculosis and abscesses. In 5 cases in which malignancy was not diagnosed, the initial cytohistology of the US-guided core biopsy or FNA was non-diagnostic (insufficient or indeterminate sample), and further studies were needed. This is clinically important, as in no patient was the initial specific diagnosis of benign lesion (which ruled out the need for a new, alternative diagnostic procedure) later found to be a false negative. A false negative would have led to a potentially more serious diagnostic error.
Nevertheless, we believe that a diagnosis of benign lesion does not rule out the need for strict clinical and radiological follow-up. This is particularly important in the case of abscesses, as these can also be present in neoplastic lesions.
The safety profile of the study procedure was also excellent. These good results could be due to various factors: (1) the procedure was performed by trained interventional pulmonologists; and (2) ultrasound is a very safe technique, because it allows the technician to puncture lesions that are in contact with the pleura, and to visualize the procedure in real time.2,12,13 CT-guided biopsy, in contrast, has a higher rate of complications,14 one of the reasons for this being the tendency to pierce or lacerate the healthy lung. “In US, you see what you are doing; in CT you see what you have done”.15
Our study has a number of limitations, some of which are related to its retrospective design. Some data, such as the type of needle used, number of punctures made, or the diameter of the lesion as measured by the ultrasound device, were missing from the preliminary procedures.
Diagnoses obtained from US-guided needle aspiration were not later confirmed using the gold standard technique (surgical biopsy) or by other diagnostic procedures (such as CT-guided biopsy) in order to avoid subjecting the patient to invasive procedures that could put them at risk. This is why, despite the intrinsic limitations, final diagnosis was established on the basis of appropriate clinical and radiological follow-up.
Another limitation to our study is the absence during the biopsy procedure of a pathologist capable of making an on-the-spot cytology assessment of the lesion. This would have reduced the number of FNA samples classified as insufficient.16–18 We recommend that a pathologist be present during the procedure to increase the diagnostic yield.
Many studies have shown ultrasound to be as effective as CT in guiding needle biopsy of peripheral pulmonary lesions, with a diagnostic yield based on confirmatory histology of between 84% and 95%, depending on the study.1,4,12,19–21
Most studies in US-guided needle biopsy have been published by interventional radiologists2,8,9; this is one of the first studies describing the results of this technique performed by interventional pulmonologists.
US imaging has a number of limitations, primarily that the entire pleural area cannot be visualized due to the presence of the ribs, and the technique is only suitable for evaluating lesions in contact with the chest wall.4,19 Therefore, only lesions with an good acoustic window can be sampled using this technique.6,12
Unlike CT, the size of the lesion does not seem to affect US-guided biopsy diagnostic yield.20–24
This is because under CT-guided puncture or needle biopsy, the lesion is not punctured in real time, unless a fluoroscopic CT scan is used, and accurate puncture of small lesions is hampered by the ribs and breathing movements. Ultrasound, however, provides dynamic real-time images, thus enabling the technician to more accurately target the lesion while viewing the tip of the needle.15 US-guided biopsy is a short, easily prepared procedure, and punctures can be repeated as required.1 In our series, as in those reported by other authors, we found that lesion size did not significantly affect diagnostic yield in US-guided biopsy.
Core biopsy specimens are larger than those obtained with needle aspiration. This not only facilitates diagnosis, but also allows pathologists to determine tumor subtypes in the case of malignancy, and to perform molecular analysis.19,20 Despite this, we found no statistically significant differences in either diagnostic yield or tumor subtype determination between FNA and core biopsy. This, however, could be attributed in part to the small size of our sample. In this study, we used different needle sizes, based on the findings of previous authors4,25 who found that needle type did not influence diagnostic yield.
Much effort has been devoted to the search for ultrasound descriptors that can be used as predictive factors to discriminate between malignant and benign peripheral pulmonary lesions. Evidence suggests that the best US criteria for characterizing these lesions include contour of the lung surface, margins when lung is aerated, destruction of normal pulmonary architecture, vascular displacement, neovascularization, and invasion of adjacent structures.6,13 Jeon et al.16 showed that the only statistically significant factor affecting diagnostic yield in US-guided transthoracic biopsy was the lesion-pleura contact arc length. Thus, with a contact arc length ≤30mm, diagnostic yield fell from 98% to 85.4%.
The use of ultrasound in respiratory medicine has increased in recent years, in part due to the development of educational programs, such as the Harmonization of Education in Respiratory Medicine for European Specialists (HERMES) initiative. The first syllabus released in connection with this project, in 2006, calls for training in ultrasound imaging techniques to be included in pulmonology training programs.26,27
Despite its advantages, US is rarely used in the study of malignant chest lesions, and in most hospitals the technique of choice is CT-guided needle biopsy.4
Scientific and technological advances in recent years have broadened the scope of application of US. One such example is the development of color Doppler, which, by preventing accidental puncture of large veins, has widened the diagnostic spectrum and improved the safety of ultrasound-guided techniques. Another new strategy involves the use of contrast agent in US, giving a clearer picture of the target tissue in peripheral lung lesions and improving diagnostic outcomes.28
Studies in larger series based on variables such as number of punctures per procedure, presence or absence of disease, lesion access, or learning curves, will give further insight into the different factors affecting diagnostic yield associated with US-guided biopsy techniques.
Meanwhile, studies such as ours illustrate the growing importance of ultrasound in anticipation of further developments that will extend its use among interventional pulmonologists.
Based on its good diagnostic yield and excellent safety profile, US-guided needle biopsy performed by a fully trained technician should be the technique of choice for the diagnosis of thoracic lesions in contact with the pleura,2 provided it is not contraindicated.
Conflict of InterestsThe authors declare they have no conflicts of interest.
Please cite this article as: García-Ortega A, Briones-Gómez A, Fabregat S, Martínez-Tomás R, Martínez-García MÁ, Cases E. Utilidad de la ecografía en el diagnóstico de lesiones torácicas periféricas realizadas en una unidad de técnicas de neumología. Arch Bronconeumol. 2016;52:244–249.