The association of systemic sarcoidosis with malignant neoplasms has been reported by several publications although there is controversy as to whether sarcoidosis predisposes patients to certain types of tumors or whether it is the tumors that cause a sarcoid reaction. The relationship between endobronchial carcinoid tumor and sarcoidosis is uncommon. We report the case of a 43-year-old male who was hospitalized due to hemoptysis. Thoracic CT revealed a pulmonary mass in the right upper lobe (RUL) as well as hypermetabolic lymphadenopathies seen with PET-CT. The endobronchial biopsies were compatible with carcinoid tumor. The patient underwent lobectomy of the RUL with mediastinal lymphadenectomy. Histology demonstrated type I endobronchial carcinoid tumor and sarcoid granulomas, both in the RUL surgical piece as well as in the mediastinal lymph nodes.
La asociación de sarcoidosis sistémica con neoplasias malignas ha sido descrita en distintas publicaciones, aunque existe controversia sobre si la sarcoidosis predispone a ciertos tipos de tumores o si son estos los que provocan una reacción sarcoidal. La relación entre tumor carcinoide endobronquial y sarcoidosis es inusual. Describimos el caso de un varón de 43 años que ingresa por hemoptisis y en el que la tomografía computarizada (TC) torácica muestra una masa pulmonar en el lóbulo superior derecho (LSD) junto con adenopatías hipermetabólicas en la tomografía por emisión de positrones (PET)-TC. Las biopsias endobronquiales fueron compatibles con tumor carcinoide. El paciente fue intervenido, practicándosele lobectomía del LSD con linfadenectomía mediastínica reglada, cuya histología demostró tumor carcinoide tipo I endobronquial y granulomas sarcoideos, tanto en la pieza de LSD como en ganglios mediastínicos.
Carcinoid tumors originate in the Kulchitsky cells of the bronchial epithelium.1,2 They are classified within the category of neuroendocrine neoplasms due to their capability to form and secrete substances.1 These pulmonary neoplasms are rare, representing less than 5% of primary pulmonary tumors.1,2 The prognosis is usually good, although some subtypes can metastasize.1 These tumors occur with the same frequency in both sexes, with a peak incidence in the fifth decade of life.1 In intrabronchial tumors, the symptoms are usually: cough, hemoptysis and recurring infections.1 Surgical resection of the tumor is the only effective treatment, fundamentally entailing lobectomy or bronchial resection.2 The factors that predispose tumor relapse are1,2: tumor size>3cm, atypical variation on histology and the presence of metastatic lymphadenopathies. The 5- and 10-year survival rates are 96% and 84%, respectively, if there is no lymph node metastasis.1
Sarcoidosis is a systemic disease characterized by the accumulation of non-caseating granulomas that distort the tissue structure of the organ where they settle and alter their function.3,4 It affects multiple organs of the body, essentially the lungs and lymph nodes, but also the skin, joints, eyes and other organs.3,4
The simultaneous coexistence of both diseases in a patient is unusual.4–7
We report the case of a patient diagnosed with pulmonary carcinoid tumor and sarcoidosis simultaneously.
Clinical ObservationsA 43-year-old male came to our emergency department due to moderate-exertion dyspnea that had been evolving over the previous 24h, accompanied by pleuritic chest pain and cough with hemoptoic expectoration (75cm2 while at home and 25cm2 observed during anamnesis). The subject is a Bolivian farmer who has been living for the last 3 years in Spain, ex-smoker for the last 5 years with a previous smoking history of 20 pack-years, with dyslipidemia treatment and no other history of interest. Physical exploration showed: BP 171/101mmHg; respiratory rate 14breaths/min; heart rate 115bpm; O2 saturation (FiO2 0.21), 91%; afebrile. Auscultation revealed crackles in the anterosuperior third of the right hemithorax. No cervical or axillary lymphadenopathies were palpated. The rest of the exploration was normal.
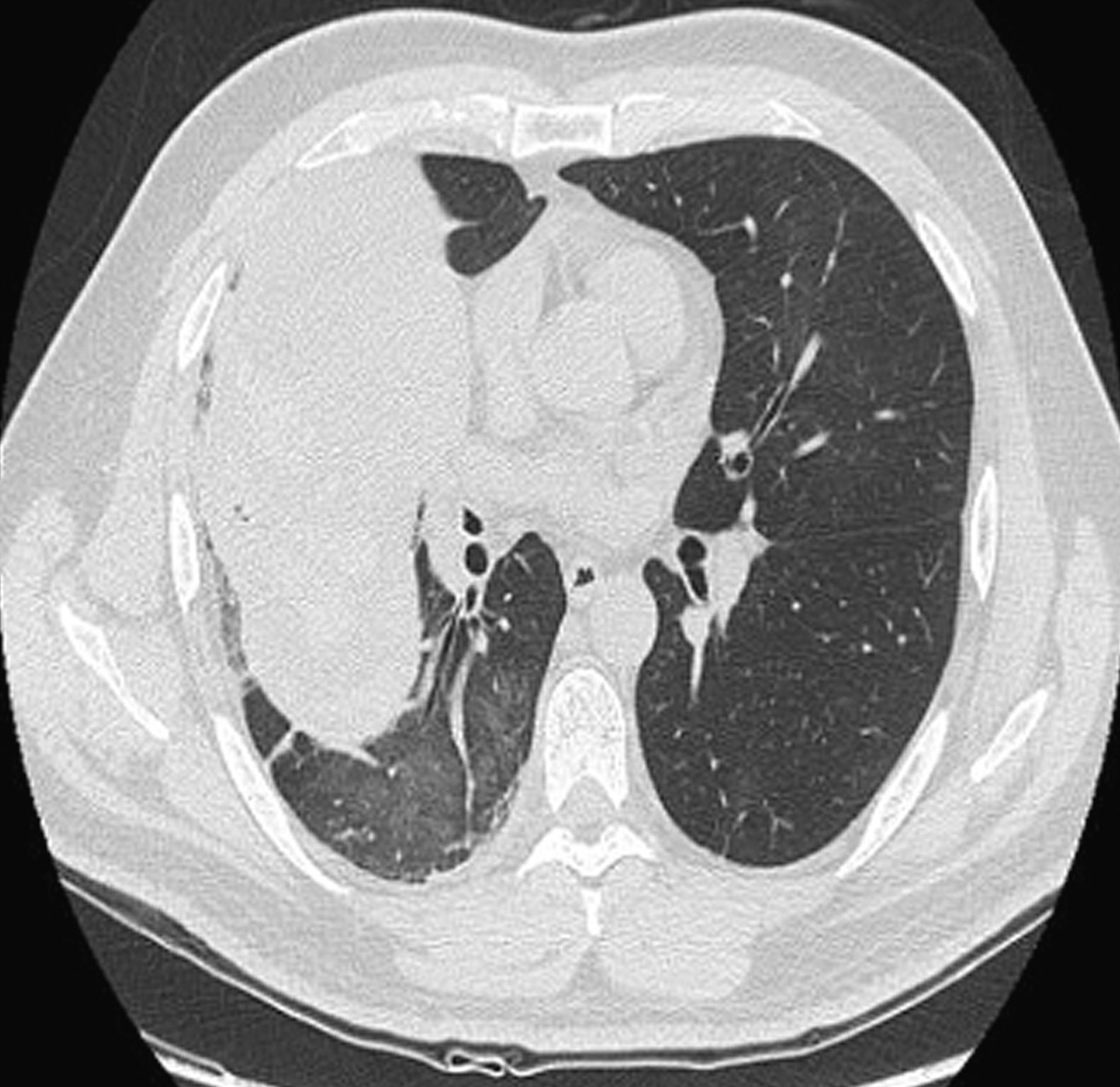
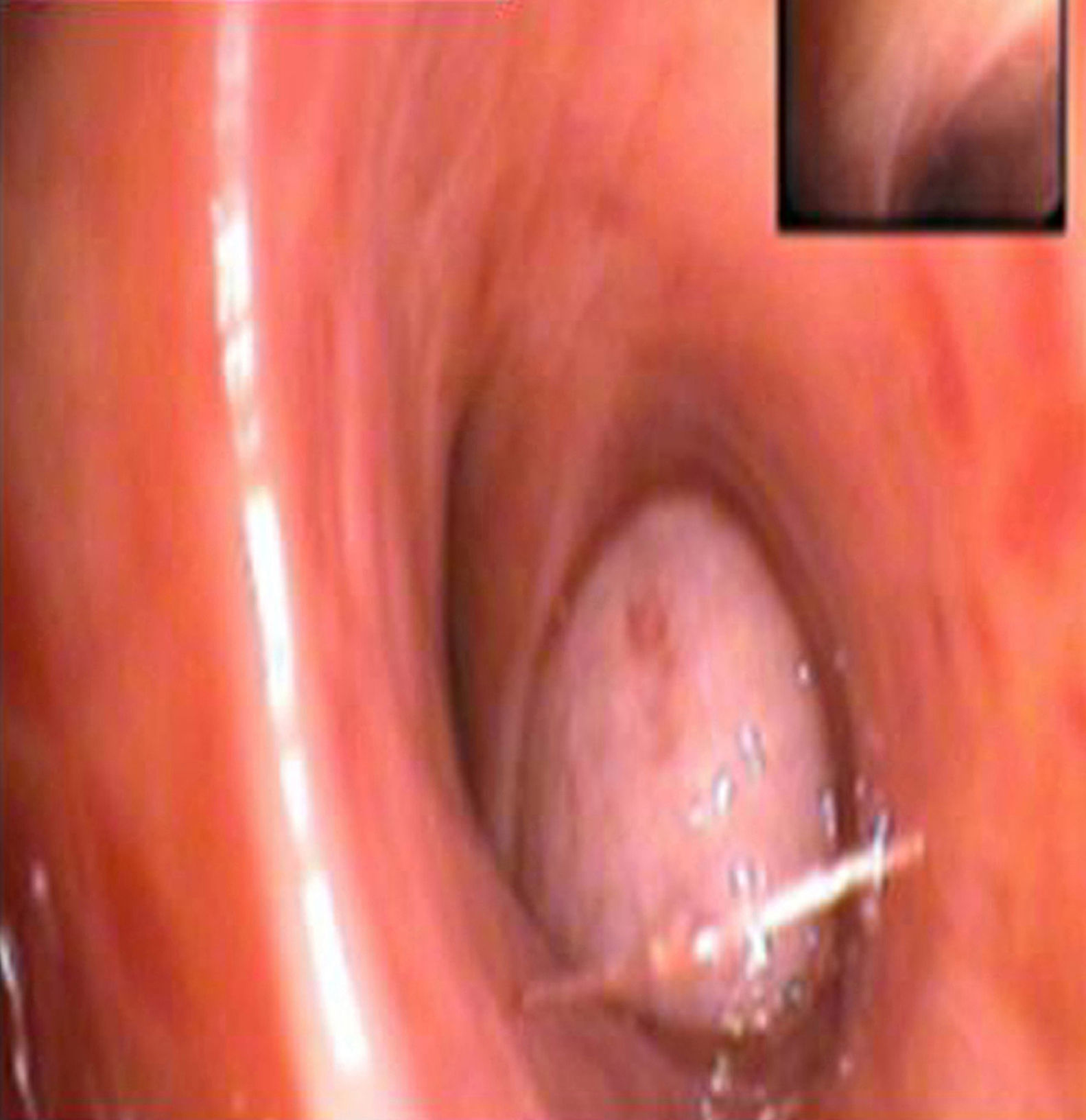
Findings in the ordinary work-up included 12,600 leucocytes, with 70% neutrophils. Sputum bacterial culture and acid-fast bacilli smear were negative. Chest radiography revealed a mass measuring 94mm×78mm×86mm in the right upper lobe (RUL) with bulging of the minor fissure and surrounding consolidation. Thoracoabdominal computed tomography (CT) (Fig. 1) demonstrated a lung mass in the RUL measuring 5.6mm×7.8mm×16.3cm, limited by the major and minor fissures, which were bulging. The mass extended up to the anterior chest wall, with no signs of infiltration, and towards the mediastinum, with no infiltration of the large blood vessels. It was accompanied by a single lymphadenopathy in the subcarinal region, with a short axis larger than 1cm. Abdominal study was normal. Flexible bronchoscopy (Fig. 2) located a smooth tumor that obstructed the entrance of the anterior segmental bronchus of the RUL. Biopsies of the endobronchial tumor were positive for carcinoid tumor.
In order to complete the staging, positron emission tomography (PET)-CT was done, where hypermetabolism was observed at the level of the RUL mass (SUV 8.9) and the mediastinum, affecting lymphadenopathies at different levels not observed on CT: right bronchial (SUV 7.1), subcarinal (SUV 5.4), retrocaval–paratracheal (SUV 4.7), right paratracheal (SUV 4.9) and right prevascular (SUV 4.5) regions.
With the diagnosis of carcinoid tumor, the patient was referred to the Department of Thoracic Surgery, where he was treated. The histological diagnosis of the surgical piece was type I carcinoid tumor and presence of sarcoid granulomas both in the lobectomy piece of the RUL as well as in the resected mediastinal lymph nodes (4R, 7, 9, 10 and 11).
The patient is currently asymptomatic. On a follow-up CT, only post-surgical changes were observed along with an upper right paratracheal lymphadenopathy that was already present in the previous study.
DiscussionPatients with sarcoidosis seem to have a higher risk of having some types of neoplastic processes (lung, stomach, small intestine, liver, melanoma, non-melanoma skin cancer, non-Hodgkin lymphoma and leukemia) according to studies published for over more than three decades.3–9 There are reports of cases in which systemic sarcoidosis and lung cancer coexisted,4,6,7 most frequently adenocarcinomas.4 The association of thoracic carcinoid tumor with sarcoidosis has also been reported.6,7 In the majority of these cases, the sarcoidosis was present before the appearance of the neoplasm, regardless of the histologic type; in others, they were detected simultaneously.4,6,7
Two possible theories try to explain the relationship between the two diseases6: the first defends that the immunological alterations that are related with active sarcoidosis may promote the development of neoplasms; the second proposes that the tumor processes may cause sarcoid reactions, which in time may evolve into systemic sarcoidosis.
It is difficult to determine whether the presence of non-caseating granulomas actually corresponds with a case of systemic sarcoidosis or with a sarcoid reaction in the context of lung cancer.4,6 In practice, the differentiation between the two entities is very difficult despite reports of some histological differences.4,10 In order to be able to talk about systemic sarcoidosis, it is necessary to confirm the presence of granulomatous lesions in at least two organs.4 In our patient, granulomatous lesions were found in the RUL lesion as well as in the mediastinal lymphadenopathies, which suggests a diagnosis of systemic sarcoidosis more than a sarcoid reaction. Granulomatous reactions11 in the heart of the tumors or in the lymph nodes that drain the tumor were reported for the first time by Hexheimer in 1917 in three breast, one colorectal and one cystic duct neoplasms, and since then similar cases have appeared in numerous publications. The coincidence of sarcoid reaction and tumor may be due to, among other causes, a reaction to a foreign body, antitumor therapy, idiopathic causes or concomitant diseases. The mechanism for forming these granulomas seems to be related with an immune response mediated by T-cells that activate the recruitment and the transformation of the monocyte-macrophage system towards epithelioid cells as a response to the persistence of tumor antigens.
In conclusion, we should keep in mind the risk for developing neoplasms in patients with diagnosis of sarcoidosis. Furthermore, we must consider the possibility that, in patients diagnosed with lung cancer who present perilesional non-caseating granulomas, we find ourselves before a true concurrent systemic sarcoidosis instead of a simple sarcoid reaction in the context of the neoformative process.
Please cite this article as: Lera Álvarez R, et al. Asociación de tumor carcinoide y sarcoidosis. Arch Bronconeumol. 2012;48:469–71.