The spectrum of pulmonary neuroendocrine cell proliferation ranges from reactive hyperplasia to small cell carcinoma and includes diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. A case is reported and features of this disease are described according to the published evidence. The entity was first included in the WHO classification of tumors in 1999 and is considered a preneoplastic condition for carcinoid tumors. Patients generally report slowly progressive dyspnea and cough, and have airflow obstruction, multiple pulmonary nodules and/or radiological signs of air trapping, although asymptomatic cases with normal pulmonary function have been described. Histologically, it is characterized by neuroendocrine cell proliferation in the airway epithelium, in most cases beyond the basal membrane forming tumorlets and carcinoid tumors that are frequently multiple. The prognosis is favorable in most cases.
El espectro de la proliferación de células neuroendocrinas pulmonares va de la hiperplasia reactiva al carcinoma de célula pequeña e incluye la hiperplasia difusa idiopática. Se describe un caso de hiperplasia difusa idiopática y se describen las características de dicha entidad según la evidencia publicada. Se trata de una entidad incluida por primera vez en la clasificación de tumores de la OMS en 1999 y considerada precursora de tumores carcinoides. Cursa con mayor frecuencia con disnea y tos, en general lentamente progresivas, obstrucción al flujo aéreo, nódulos pulmonares múltiples y signos radiológicos de atrapamiento aéreo, aunque se han descrito casos asintomáticos sin afectación funcional. Histológicamente se caracteriza por la proliferación de células neuroendocrinas en el epitelio de las vías respiratorias, en muchos casos más allá de la membrana basal formando tumorlets y tumores carcinoides, con frecuencia múltiples. Su pronóstico es favorable en la mayoría de los casos.
The spectrum of cell proliferation with neuroendocrine (NE) differentiation goes from the hyperplasia of NE cells to small-cell carcinoma.1 The term tumorlet designates an aggregate of NE cells with a similar morphology to carcinoid tumor and a diameter less than 5mm.1 Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) is a rare entity characterized by generalized, extensive hyperplasia of NE cells in the peripheral airway without any primary process to justify it, and it is considered a precursor entity of carcinoid tumors.2 We report a case with multiple synchronous carcinoid tumors and a review of the literature.
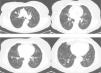
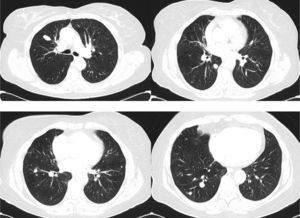
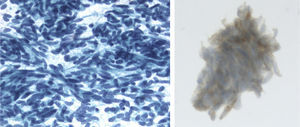
Clinical ObservationThe patient is a 73-year-old woman with no history of interest. Pre-operative chest radiography demonstrated several lung nodules, although the patient was asymptomatic. Hemogram and biochemical determination in serum and urine were normal. Thoracic CT showed multiple bilateral lung nodules of varying sizes between 1 and 10mm, and two larger nodules, one measuring 20mm in the right upper lobe and another measuring 12mm in the right lower lobe (Fig. 1). Lung function tests were normal. Studies searching for a primary neoplastic process, including abdominal/pelvic CT, gynecological assessment and PET/CT, were negative. Endoscopically, the bronchial tree did not present significant alterations, and the cytological and microbiological studies of the bronchoscopic samples (bronchial brushing and suction, and bronchoalveolar lavage) were negative. Fine-needle aspiration of the larger nodule revealed a low-grade fusocellular tumor and neuroendocrine immunophenotype (positive pankeratin, chromogranin and CD56, and negative vimentin and S-100; Fig. 2). Given the multifocal nature of the lesions, we considered the differential diagnosis with metastasis of a mesenchymal neoplasm, melanoma or carcinosarcoma. Video-assisted thoracoscopy provided biopsy samples that showed two carcinoid tumorlets measuring 4 and 3mm in diameter. Immunohistochemistry was positive for CD-56 and TTF-1, and the cell proliferation index (Ki67) was less than 2%. Due to the multiplicity of the process, surgical treatment was ruled out, and chemotherapy was also not considered adequate given the proliferation index. Thus, it was decided to observe the patient. Twenty-four months later, the patient continued to be asymptomatic, with normal lung function, and most of the nodules remained stable, except for minimal growth seen in the two of the largest (3 and 4mm).
Material from the fine-needle aspiration, fixed in alcohol and stained with Papanicolaou. Very cellular frotis with abundant loose cells. The cells are monotone with regular, oval and round nuclei, granular chromatin without nucleolus. Immunocytochemistry: positive chromogranin (cytoplasmic and granular).
NE cells are situated at the base of the bronchial and bronchiolar mucosa. The spectrum of NE cell proliferation goes from hyperplasia to small-cell carcinoma (Table 1).1 NE cell hyperplasia can be seen as a linear matrix of NE cells or accumulations of these throughout the respiratory epithelium. The proliferation can go beyond the basal membrane, forming nodular aggregates of NE cells with morphology similar to that of carcinoid tumors with a diameter of less than 5mm, which are called tumorlets. The first descriptions of hyperplasia and tumorlets were casual histological findings from the study of surgical pieces that had been obtained due to several chronic pulmonary pathologies like abscesses, bronchiectasis, cystic fibrosis, tuberculosis and even chronic bronchitis and emphysema, and they were therefore considered a reactive process associated with the regeneration of the airways and alveoli with chronic damage.1,3,10 In 1992, Aguayo et al.3 described six patients with no history of tobacco use, with the same clinical, radiological and functional characteristics and a histological substrate of generalized NE cell hyperplasia. They believed them to be a separate entity, which they called DIPNECH. Largely based on this series, but also on other later descriptions,4–6 the WHO included it in the tumor classification of 1999.7
Spectrum of Pulmonary Neuroendocrine Neoplasms and Proliferation.
| Hyperplasia of NE cells and tumorlet |
| NE cell hyperplasia |
| NE cell hyperplasia associated with fibrosis and/or inflammation |
| NE cell hyperplasia adjacent to carcinoid tumors |
| Diffuse idiopathic neuroendocrine cell hyperplasia with or without obstruction and/or fibrosis of the airway |
| Tumorlets |
| Tumors with NE morphology |
| Typical carcinoid |
| Atypical carcinoid |
| Large-cell NE carcinoma |
| Small-cell carcinoma |
| Non-small cell carcinomas with NE differentiation |
| Other tumors with NE properties |
| Pulmonary blastoma |
| Primitive neuroectodermal tumor |
| Desmoplastic small round cell tumor |
| Carcinoma with rhabdoid phenotype |
| Paraganglioma |
NE: neuroendocrine. Adapted from Travis.1
DIPNECH is more frequent in women. Davies et al.8 described a series of 19 patients, 15 (79%) of whom were women. Among the 25 cases reported in a systematic search of cases from 2004 to 2010, 23 (92%) were seen in women.9 The mean age at diagnosis was about 60, with a wide age range (22–79), and it is more frequent in patients without a history of smoking.3,6,8,9 The epidemiological characteristics of the case described in a 73-year-old never-smoker woman meet these descriptions.
Clinically, symptoms include dyspnea, cough and wheezing. The evolution time is variable, although it is generally long.9 There may have been a previous diagnosis made years earlier, frequently of bronchial asthma.10,11 Most cases described present this symptomatology, but in the series by Davies et al.8 10 of the 19 patients were asymptomatic. There is frequently airflow obstruction or a mixed alteration, but it is not rare for lung function to be normal.8–10 Lung nodules are the most frequent radiological manifestation, followed by ground glass attenuation, mosaic pattern due to air trapping secondary to constrictive bronchiolitis caused by hyperplasia, nodular thickening of the bronchial walls and bronchiectasis.12,13 In the case described, the patient was asymptomatic, lung function was normal and CT showed numerous bilateral lung nodules, so the initial suspected diagnosis was metastasis of an unknown primary tumor. In the series by Davies et al.,8 out of the 10 asymptomatic patients, 8 presented previous neoplasm.
Histologically, DIPNECH is characterized by the generalized proliferation of disperse individual cells, small nodules (NE bodies) or linear proliferations of NE cells limited to the bronchial and bronchiolar epithelium.2 Most cases require surgical biopsy for diagnosis, but with the presence of compatible symptoms, function and radiology, the presence of NE cell hyperplasia in a transbronchial biopsy could be considered sufficient.9 In the resected piece, some airways may appear normal and others minimally altered and ecstatic, with no evidence of NE cell hyperplasia in the evaluated sections, and therefore the surgical biopsy may sometimes not show evidence of NE cell hyperplasia.9 In the case described, in addition to the tumorlet, the evaluated pulmonary parenchyma only showed minimal alterations and mucus plugging (a finding present in more than 50% of the series by Davies et al.8) with no other evidence of NE cell hyperplasia. Furthermore, the differentiation between the reactive proliferation of NE cells, including its casual finding, and DIPNECH may be difficult, as the histological findings are alike. But, diffuse extension in an adequate tissue sample and/or the presence of altered lung function, evidence of air trapping on CT and/or multiple lung nodules, would be able to confirm DIPNECH.1 Recently, differences have been reported in the antigen expression profile of NE cells from reactive proliferations compared with that of DIPNECH.14
The presence of typical carcinoid tumors in DIPNECH is very frequent; in the series by Davies et al.,8 47%, and in the review by Nassar et al.,9 40%. Inversely, the presence of NE cell hyperplasia and tumorlets in surgically-resected pieces with carcinoid tumors is also frequent. Miller et al. found this to be true in 76% of a series of 25 patients with resected peripheral carcinoid tumors,6 while Aubry et al.15 repeated the same finding in 77% of 28 patients with two or more tumorlets or resected carcinoid tumors. With all this in mind, the WHO considers DIPNECH to be an entity that is a precursor of carcinoid tumors and included it in the tumor classification of 1999.7 Later studies have supported this.16,17
In most cases, the evolution is favorable with clinical, functional and radiological stability for years.8,9 Despite the minimal growth of two of the tumors, it can be considered that the case described has behaved this way during the 24-month follow-up to date. There have been reports of airflow obstruction progressing to respiratory failure4,8,18 as well as reports of metastasis, although these cases are rare.19
In spite of DIPNECH being a proliferative process, there is no consistent evidence to support treatment with chemotherapy. In sporadic cases in which it has been used, the result was unfavorable.3 In patients with airflow obstruction, the therapeutic options center around en an assay with steroids, inhaled or systemic, and the most recommendable option in asymptomatic patients with normal lung function is observation.8,9 In cases of associated carcinoid tumors, surgical resection may be considered, and its prognosis is similar to that of resected solitary carcinoid tumors.16
In conclusion, diffuse idiopathic pulmonary neuroendocrine cell hyperplasia is a rare entity that is recently being reported with greater frequency. Despite the fact that the majority of the cases described are symptomatic patients with lung function alterations, it should also be included in the differential diagnosis of asymptomatic patients with normal lung function and compatible radiological alterations, especially in middle-aged women with no former history of smoking.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Montoro Zulueta FJ, et al. Hiperplasia difusa idiopática de células neuroendocrinas pulmonares con tumores carcinoides múltiples sincrónicos. Arch Bronconeumol. 2012;48:472–5.