Recent epidemiological evidence suggests that asthma, particularly type 2 high asthma characterized by airway eosinophilia, may confer a protective effect against severe outcomes of COVID-19.1–3 Eosinophils, traditionally associated with allergic responses and helminth infections, have demonstrated also antiviral and antibacterial properties. This study aimed to investigate the prognostic value of pre-existing absolute eosinophil count (AEC) in mortality associated with bacterial pneumonia, COVID-19 infection, and influenza. Utilizing data from a large healthcare provider in Israel, over 77,000 COVID-19 patients, 47,000 pneumonia patients, and 5000 influenza patients were included.
Eosinophils contribute to both antiviral and antibacterial immunity by utilizing specialized proteins with clear antiviral and antibacterial properties, along with the ability to generate extracellular DNA traps.4 A recent study has demonstrated an inverse relationship between eosinophils and influenza PCR positivity in COPD patients.5 Eosinophils have also been found to have an anti-viral effect in animal models by decreasing viral replication and increasing viral clearance from the airways.6–8 It is interesting to note that eosinophils in lymphoid tissues of mice infected with influenza modified the surface expression of activation markers of immune cells.9
In the present study we sought to examine pre-existing absolute eosinophilic count (AEC) as a prognostic marker for mortality in bacterial pneumonia and in respiratory infections due to SARS-CoV-2, influenza. We used the computerized database of the Clalit Health Services, the largest healthcare provider in Israel to identify three study groups; (i) PCR confirmed SARS-CoV-2 infection between 1.3.2020 and 18.12.2020, (ii) Hospitalized patients with PCR confirmed influenza between 1.1.2015 and 31.12.2019 and (iii) Hospitalized patients with bacterial pneumonia, identified based on ICD-9 codes (481–483), between 1.1.2015 and 31.12.2019. An AEC of 150 was selected as the cutoff to determine the role of eosinophils in the respiratory immune response. This choice is based on the definition of high type 2 (T2) status, which is indicated by an AEC≥150cells/μl10 and is associated with airway eosinophilia.11
Within each cohort, the risk of 30-days all-cause mortality was estimated in those with AEC≥150cells/μl and compared to those with AEC<150cells/μl. The Eosinophils level closest to the diagnosis within the range of 14–365 days prior to the diagnosis was selected to mitigate the potential impact of acute disease influences. The study was approved by the Clalit Health Services institutional review board and was exempt from the requirement for informed consent. Cox proportional hazard regression models were used to assess the association between pre-existing AEC (≤150cells/μl vs. >150cells/μl) and mortality within 30 days in each study group separately. The multivariable Cox regression models were adjusted for age, gender, ethnicity, socioeconomic status, obesity (BMI>30), smoking, diabetes, hypertension, ischemic heart disease, chronic heart failure, arterial fibrillation, malignancy, cerebrovascular diseases, chronic lung diseases, vascular diseases, chronic renal failure, OCS (oral corticosteroid) use in the previous year (at least 6 prescriptions of oral corticosteroid use in the previous year).
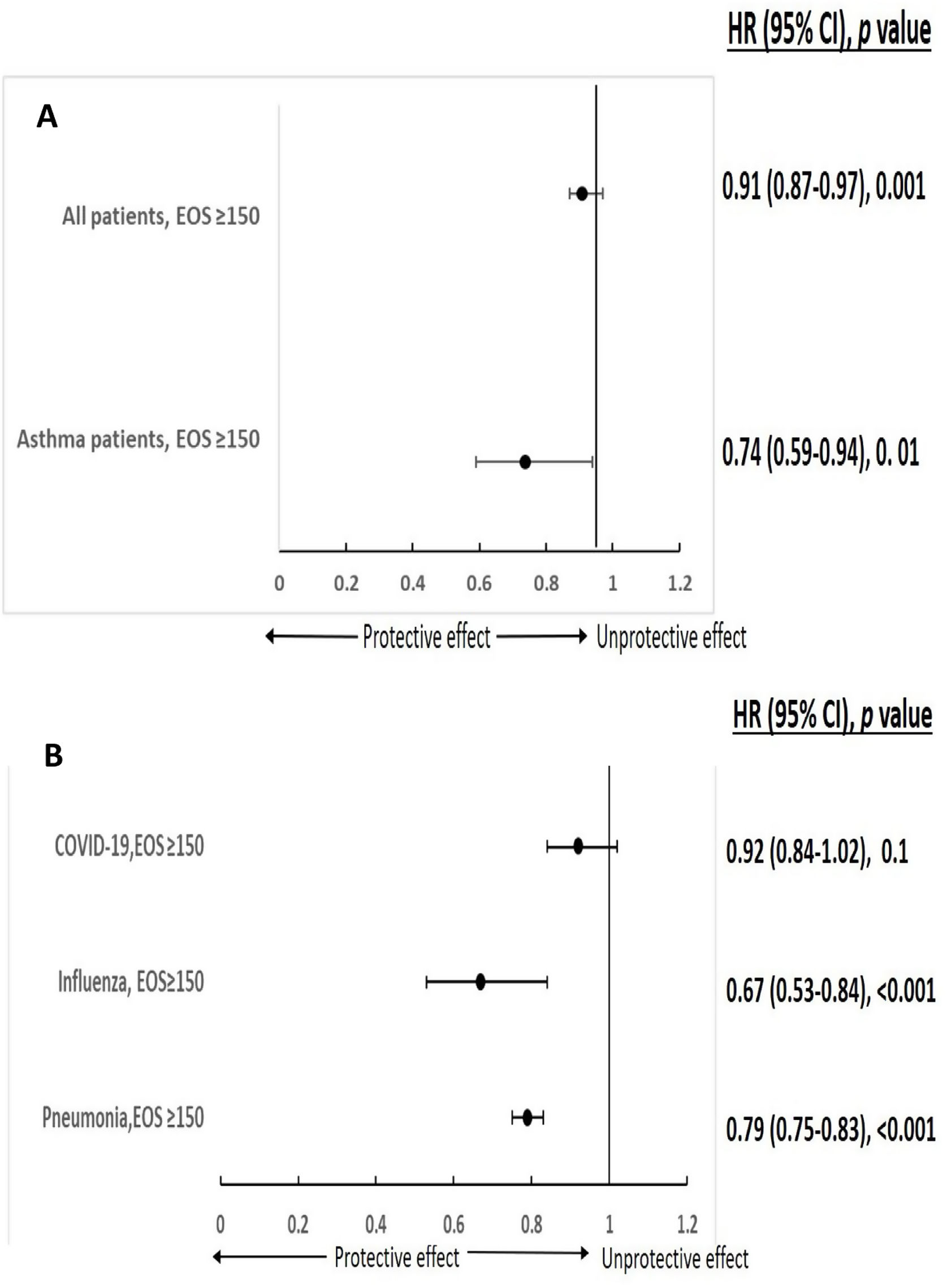
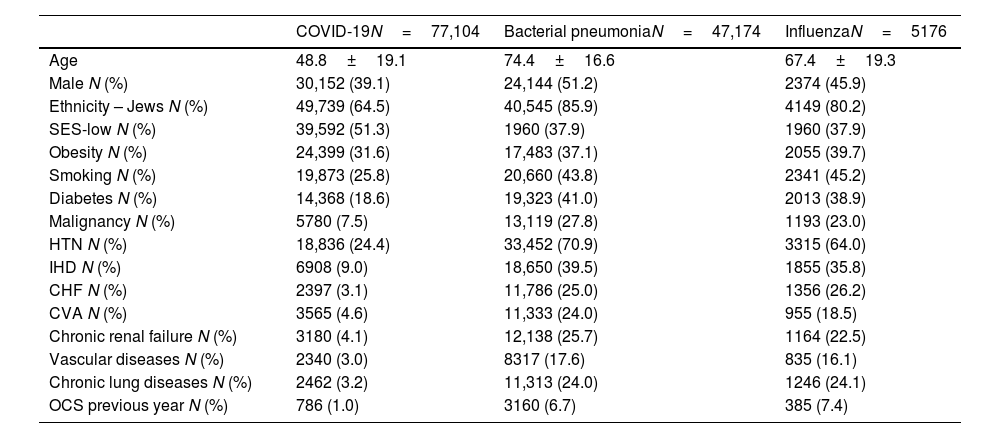
A total of 77,104 patients with COVID-19, 47,174 with bacterial pneumonia and 5176 patients with influenza were included in the study. 6.8% (5260/77,104) of COVID-19 was defined with moderate/severe disease. Demographic and clinical characteristics of 3 study groups are presented in Table 1. As systemic steroids can significantly influence eosinophil count, it is noteworthy that only a minority of patients used OCS in the last year in all three groups (1%, 6.7%, and 7.4% for COVID-19, bacterial pneumonia, and influenza patients, respectively). The comorbidities outlined in Table 1 can significantly influence the course and severity of respiratory infections. Conditions such as cardiovascular disease, diabetes, and chronic respiratory diseases can impair immune function, exacerbate inflammation, and increase susceptibility to respiratory pathogens, leading to heightened morbidity and mortality rates.12 Notably, clustered comorbidities carry greater significance than single conditions; hospitalized COVID-19 patients with multimorbidity face heightened mortality risks compared to those without.13 To mitigate the influence of comorbidities, we employed multivariable analysis in our study. In multivariable analysis, pre-existing AEC≥150 in COVID-19 infection was significantly associated with a reduced risk for moderate to severe disease in both asthmatic patients and the general study population (HR 0.74 (0.59–0.94), p=0.01; HR 0.91 (0.87–0.97), p=0.001, respectively) (Fig. 1A). Furthermore, pre-existing AEC≥150cells/μl was linked to a significantly decreased risk of 30-days mortality in patients hospitalized with influenza or bacterial pneumonia (HR 0.67 (95% CI 0.53–0.84), p<0.001; HR 0.79 (95% CI 0.75–0.83), p<0.001, respectively), with a trend toward reduced mortality in COVID-19 (HR 0.92 (95% CI 0.84–1.02), p=0.1) (Fig. 1B).
Demographic and clinical characteristics of study population.
| COVID-19N=77,104 | Bacterial pneumoniaN=47,174 | InfluenzaN=5176 | |
|---|---|---|---|
| Age | 48.8±19.1 | 74.4±16.6 | 67.4±19.3 |
| Male N (%) | 30,152 (39.1) | 24,144 (51.2) | 2374 (45.9) |
| Ethnicity – Jews N (%) | 49,739 (64.5) | 40,545 (85.9) | 4149 (80.2) |
| SES-low N (%) | 39,592 (51.3) | 1960 (37.9) | 1960 (37.9) |
| Obesity N (%) | 24,399 (31.6) | 17,483 (37.1) | 2055 (39.7) |
| Smoking N (%) | 19,873 (25.8) | 20,660 (43.8) | 2341 (45.2) |
| Diabetes N (%) | 14,368 (18.6) | 19,323 (41.0) | 2013 (38.9) |
| Malignancy N (%) | 5780 (7.5) | 13,119 (27.8) | 1193 (23.0) |
| HTN N (%) | 18,836 (24.4) | 33,452 (70.9) | 3315 (64.0) |
| IHD N (%) | 6908 (9.0) | 18,650 (39.5) | 1855 (35.8) |
| CHF N (%) | 2397 (3.1) | 11,786 (25.0) | 1356 (26.2) |
| CVA N (%) | 3565 (4.6) | 11,333 (24.0) | 955 (18.5) |
| Chronic renal failure N (%) | 3180 (4.1) | 12,138 (25.7) | 1164 (22.5) |
| Vascular diseases N (%) | 2340 (3.0) | 8317 (17.6) | 835 (16.1) |
| Chronic lung diseases N (%) | 2462 (3.2) | 11,313 (24.0) | 1246 (24.1) |
| OCS previous year N (%) | 786 (1.0) | 3160 (6.7) | 385 (7.4) |
SES, socioeconomic status; Obesity, BMI>30; HTN, hypertension; IHD, ischemic heart disease; CHF, congestive heart failure; CVA, cerebral vascular accident; Chronic lung diseases, Chronic bronchitis, COPD, Bronchiectasis; OCS, oral corticosteroids≥6 prescriptions per year.
Eosinophils (EOS) and respiratory infection severity and mortality (A – EOS and COVID-19 severity, B – EOS and 30 days mortality). *Adjusted: age, gender, ethnicity, SES, obesity, smoking malignancy, diabetes, IHD, CHF, AF, HTN, CVA, lung diseases, vascular diseases, chronic renal failure, steroid previous year.
Our data further support that patient with preexisting AEC>150cells/μl had reduced risk of mortality due to viral or bacterial respiratory infections. These associations support the concept that eosinophils have a protective role in viral or bacterial respiratory infections. Specifically, T2 high inflammation is associated with reduced COVID-19 binding capacity, as it lowers the expression of the host ACE2 receptor gene. Consequently, this correlates with a diminished ability of the virus to enter respiratory epithelial cells.14 It has been reported that preexisting eosinophilia≥150cells/μl in asthma patients was protective from COVID-19 hospitalization. It was further demonstrated that asthma patients with eosinophils≥150cells/μl during hospitalization due to COVID-19 had lower mortality rates.15 Of note, patients with severe COVID-19 who had AEC<150cells/μl were more likely to develop acute respiratory worsening and resolution in eosinopenia was related to clinical improvement.15
The eosinophil immune response occurs through its recognition of fungi, bacteria, and viruses which are recognized by several different molecules, including the retinoic acid-inducible gene 1 and the toll-like receptors.16 The immune protection provided by eosinophils is mediated by multiple mechanisms and properties. Several cytoplasmatic granules in eosinophils secrete ribonucleases (e.g., eosinophil-derived neurotoxins and eosinophil cationic proteins), nitric oxide and cytokines, which promote antigen presentation and enhance CD8+ T cell response. Eosinophils are not only competent effector cells, but also antigen-presenting cells, which enable them to engage in a wide range of immune responses.17 In vitro and animal studies, have demonstrated that eosinophils are capable of responding to rhinovirus, respiratory syncytial virus, influenza A, parainfluenza virus and pneumonia virus of mice.5 When transferred into influenza-infected mice, eosinophils upregulate antigen presenting markers (MHCI and CD86), interact directly with CD8+ T cells, and increase the recruitment of virus-specific CD8+ T cells into the lung to promote antiviral immunity.18 Eosinophils also have antibacterial properties through their phagocytic functions and extracellular trap formation.16
This study's main limitation stems from its reliance on computerized databases and ICD codes for patient diagnoses, which may result in missing data, misclassifications, or coding inaccuracies, potentially affecting patient identification and categorization.
Preexisting high eosinophil blood levels are associated with decreased mortality in viral and bacterial infection in the general population. These findings support the notion that eosinophils play a protective role in respiratory infections, potentially through their antiviral and antibacterial properties. Further prospective studies are warranted to elucidate the mechanisms underlying eosinophil-mediated immunity in respiratory infections
FundingThe research has no funding sources.
Conflict of interestsThe authors state that they have no conflict of interests.