Venous thromboembolism (VTE) comprises deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE). PTE is the third leading cause of cardiovascular mortality, following stroke and acute myocardial infarction, and has a major socioeconomic impact.1 Anticoagulation is the treatment of choice in both the acute phase and follow-up of PTE patients, although there are clinical situations in which contraindications prevent its use.2 When anticoagulant therapy is contraindicated in patients with VTE,2 for example, vena cava filters (VCF) are indicated. However, evidence supporting the efficacy and safety of VCFs is based on 2 clinical trials3–5 and a systematic review and meta-analysis showing that VCF placement was associated with a 50% decline in PTE and an increase in DVT of approximately 70%, but had no effect on all-cause mortality or PTE-related mortality.6
The scarcity of information in the literature has prompted many authors to conduct studies to analyze the safety and efficacy of VCFs, although insufficient conclusions have been reached to indicate their use with a high level of evidence.6 For this reason, we aimed to analyze both short-term (major bleeding, recurrence and death at 30 days) and long-term complications in patients treated at our center, and to compare these results with a previously published historical series.7
We performed a retrospective, single-center study of consecutive patients with symptomatic acute PTE who underwent VCF placement between January 2015 and August 2019. We analyzed the rate of complications (major bleeding, recurrence, and death) at 30 days, and compared our results with those previously published by Muriel et al.7 using the Z-test to compare proportions.
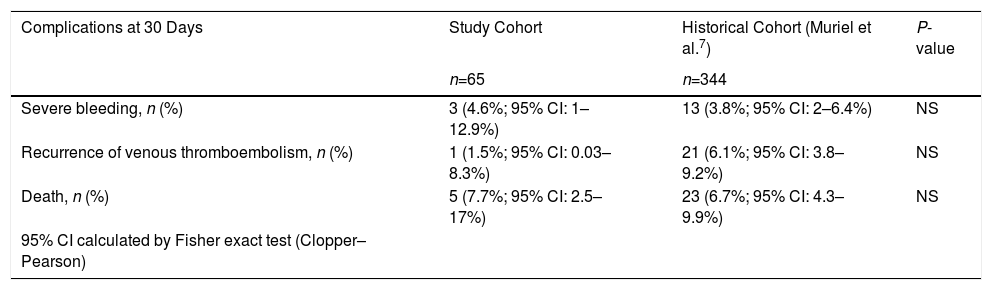
During the study period, 65 inferior VCFs were placed in patients with a median age of 62 years, mainly men (63.5%). The most frequent comorbidities were cancer (44.6%), smoking (32.3%), and dyslipidemia (27.7%). The reasons for VCF placement were surgical intervention (53.8%), recent bleeding (38%), and high risk of bleeding (18.5%). The median time from VTE to VCF placement was 14 days. In approximately two-thirds of the patients, the VCF could be withdrawn without incident. At 30 days, there was 1 recurrence (1.5%), 3 severe bleeding events (4.6%), and 5 deaths (7.7%). Median follow-up was 8.77 months. Throughout the follow-up period there were 2 recurrences (3.1%), 5 severe hemorrhages (7.7%), and 15 deaths (23.1%) (Table 1 Supplementary material). There were no statistically significant differences between complications at 30 days in our series and those reported by Muriel et al.7 (Table 1).
Comparison of Complications at 30 Days in Patients with Venous Thromboembolism Treated with Inferior Vena Cava Filter.
| Complications at 30 Days | Study Cohort | Historical Cohort (Muriel et al.7) | P-value |
|---|---|---|---|
| n=65 | n=344 | ||
| Severe bleeding, n (%) | 3 (4.6%; 95% CI: 1–12.9%) | 13 (3.8%; 95% CI: 2–6.4%) | NS |
| Recurrence of venous thromboembolism, n (%) | 1 (1.5%; 95% CI: 0.03–8.3%) | 21 (6.1%; 95% CI: 3.8–9.2%) | NS |
| Death, n (%) | 5 (7.7%; 95% CI: 2.5–17%) | 23 (6.7%; 95% CI: 4.3–9.9%) | NS |
| 95% CI calculated by Fisher exact test (Clopper–Pearson) |
CI: confidence interval; NS: not significant.
Source: Study cohort versus previously published cohort.7
In our series, VCF placement in patients with VTE with a contraindication for anticoagulant therapy was effective and safe, and the complication rate was similar to that of previous publications. The effectiveness of VCFs has always been a source of controversy, and for that reason studies are needed to support their effectiveness and safety. Decousus, in the Prévention du Risque d’Embolie Pulmonaire par Interruption Cave trial (PREPIC4) trial, initiated what has become the routine use of VCF in clinical practice, demonstrating its efficacy in the prevention of PTE. The study was an open-label randomized trial of 400 participants with documented DVT or PTE receiving anticoagulation with an 8-year follow-up that demonstrated that permanent VCF placement decreased the incidence of PTE during follow-up. However, one of the reasons why the effectiveness of these devices is still under discussion is that there was no reduction in overall mortality; it should be mentioned, however, that the study series was an elderly population with significant risk factors, such as concomitant cancer and cardiovascular disease. In spite of this, the use of VCF in highly selected patients is supported by the major scientific societies and specified in the main clinical practice guidelines2 as a strategy for PTE prevention in patients with a contraindication for anticoagulation. It is precisely in this group of highly selected patients with a high risk of bleeding that other studies have tried to analyze the possible disadvantages of VCF by examining short-term complications, and this was the main goal of our study. Muriel et al.7 showed that although patients treated with VCF had a higher risk of bleeding, major bleeding was not statistically significantly higher than in a control group treated with anticoagulant therapy. These are exactly the findings that we wanted to compare in our analysis, and indeed, we found a complication rate similar to that of Muriel et al.7 in our study: 30-day mortality was 7.7% and 30-day severe bleeding was 4.6%.
Our study has some limitations: it was performed in a single center and data were collected retrospectively. Even so, the number of VCFs analyzed is high, and a registry is available in our hospital that allows all patients who received a VCF to be analyzed consecutively. Comparing our results with an external cohort of patients has shown a similar complication rate. Another limitation is the size of the sample, as it does not allow for sub-group analysis.
The interest of this scientific letter lies in our analysis of the indications and contraindications of VCF placement in patients with VTE and contraindication for anticoagulant treatment, revealing a similar complication rate in our series to one published previously.
Conflict of InterestsLuis Jara Palomares has received honoraria for speaking engagements and for travel and accommodation from Rovi, Pfizer, Menarini, Leo-Pharma and GSK, unrelated to this manuscript. Maria Isabel Asensio Cruz has received honoraria for travel and accommodation from Rovi, Novartis and Teva, unrelated to this manuscript. Raquel Morillo Guerrero has received honoraria for speaking engagements and for travel and accommodation from Pfizer, Menarini and GSK, unrelated to this manuscript. The other authors state that they have no conflict of interests.
Please cite this article as: Lopez-Ruz S, Marin Romero S, Elias Hernandez T, Asensio Cruz MI, Barca-Hernando M, Montero Romero E, et al. Análisis de las indicaciones y complicaciones de los pacientes con enfermedad tromboembólica venosa a los que se ha colocado filtro de vena cava inferior. Arch Bronconeumol. 2021;57:444–445.