With an estimated 2.2 million new cancer cases and 1.8 million deaths in 2020, lung cancer is the second most commonly diagnosed cancer and the leading cause of cancer death.1 According to the clinical histologic characteristics, lung cancer is mainly divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), which make up 85% of all cases.2 The common subtypes of NSCLC mainly include lung adenocarcinoma, lung squamous cell carcinoma and large cell carcinoma.
The role of the immune system in tumorigenesis has become increasingly appreciated, such that immune evasion is now considered one of the hallmarks of cancer.3–5 Macrophages comprise the bulk of the immune infiltrate in tumours and are the key cell type that links inflammation and cancer.6 They are extremely plastic cells capable of diverse functions, with their distinct phenotypes or subpopulations: M1 or classically activated macrophages and M2 or alternatively activated macrophages.7,8 Differentiation of M1 macrophages occur in the presence of Toll-like receptor activation in the presence of Th1 helper cytokines and is involved in the Th1 response to pathogens, they are excellent antigen-presenting cells resulting in immunostimulation and accelerate the activation of adaptative immune responses.7,8 In contrast, Th2 cytokines have been shown to induce M2 macrophage polarization and participate in the Th2 immune response. M2 macrophages are characterized by impaired ability to present antigen and function to scavenge debris and promote angiogenesis, tissue remodelling, and repair.7,8 Specifically in cancer, it is believed that M1 support initiation of tumorigenesis whereas M2 are typically associated with tumour progression.7,8 Here, we study the alveolar macrophage populations in bronchoalveolar lavage (BAL) samples from patients with different types of lung cancer.
From November 2021 to October 2022, we collected BAL samples from adults’ patients with diagnostic of primary lung cancer (none of the patients had yet received treatment) and control subjects at the two hospitals. Patients with active infection or immunosuppressed were excluded. BAL was performed at the time of diagnostic fibreoptic bronchoscopy using warm sterile 0.15M NaCl instilled in 50ml aliquots to a total volume of not greater than 150ml. In patients with lung cancer, lavage was performed in the bronchus closest to the tumour lesion. The endobronchial findings in all control subjects were normal, and in this group either the right middle lobe or the lingula lobe was lavaged. An aliquot of 10ml of BAL was immediately processed for immunological study. After centrifugation, macrophages were labelled to discern between M1 and M2 populations with antibodies for flow cytometry (αCD14-APC and αCD86-FITC for M1, αCD206-PE and αCD163-PE-Cy7 for M2). The data was analyzed with FlowJo® (BD Bioscience®). Sociodemographic data, smoking, final diagnosis, tumour stage, treatments received and survival were collected as covariates. Staging was performed using the current system of the 8th Edition of the TNM Classification of Lung Cancer.9 Ethics committee approval of the two centres was received and informed consent of all participating subjects was obtained. Quantitative variables were described using median (interquartile range), and qualitative variables were described using absolute and relative frequencies. Comparisons were conducted using Mann–Whitney U in the case of quantitative variables, and Fisher's exact test for qualitative variables. For multiple comparisons, Kruskal–Wallis test was used. All data analysis was performed using SPSS 24 (SPSS, IBM Corp.).
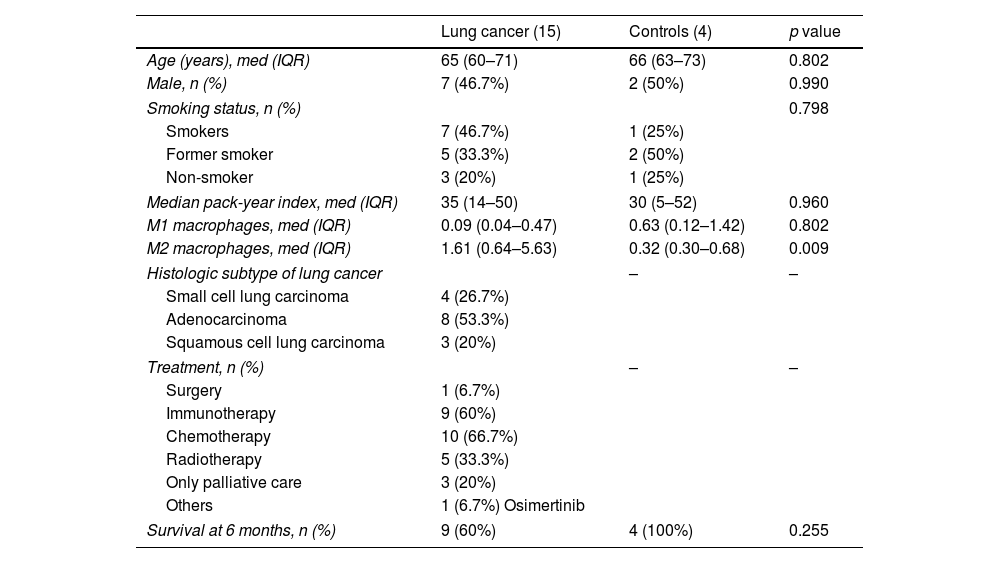
We included 16 patients with lung cancer with a median age of 65 years and 4 patients with benign non-infectious pathology (3 with interstitial lung disease and with 1 tracheal stenosis) as the control group, with median age of 66 years. In the group of patients with lung cancer, the most frequent histologic subtype was adenocarcinoma (53.3%) and most of the cases were stage IV (86.6%). The main results of the enrolled patients are shown in Table 1. For the final data analysis, patient 10 was excluded from the study because he changes his place of residence and was lost to follow-up.
Baseline characteristics of the patients included in the study.
| Lung cancer (15) | Controls (4) | p value | |
|---|---|---|---|
| Age (years), med (IQR) | 65 (60–71) | 66 (63–73) | 0.802 |
| Male, n (%) | 7 (46.7%) | 2 (50%) | 0.990 |
| Smoking status, n (%) | 0.798 | ||
| Smokers | 7 (46.7%) | 1 (25%) | |
| Former smoker | 5 (33.3%) | 2 (50%) | |
| Non-smoker | 3 (20%) | 1 (25%) | |
| Median pack-year index, med (IQR) | 35 (14–50) | 30 (5–52) | 0.960 |
| M1 macrophages, med (IQR) | 0.09 (0.04–0.47) | 0.63 (0.12–1.42) | 0.802 |
| M2 macrophages, med (IQR) | 1.61 (0.64–5.63) | 0.32 (0.30–0.68) | 0.009 |
| Histologic subtype of lung cancer | – | – | |
| Small cell lung carcinoma | 4 (26.7%) | ||
| Adenocarcinoma | 8 (53.3%) | ||
| Squamous cell lung carcinoma | 3 (20%) | ||
| Treatment, n (%) | – | – | |
| Surgery | 1 (6.7%) | ||
| Immunotherapy | 9 (60%) | ||
| Chemotherapy | 10 (66.7%) | ||
| Radiotherapy | 5 (33.3%) | ||
| Only palliative care | 3 (20%) | ||
| Others | 1 (6.7%) Osimertinib | ||
| Survival at 6 months, n (%) | 9 (60%) | 4 (100%) | 0.255 |
Med: median; IQR: interquartile range.
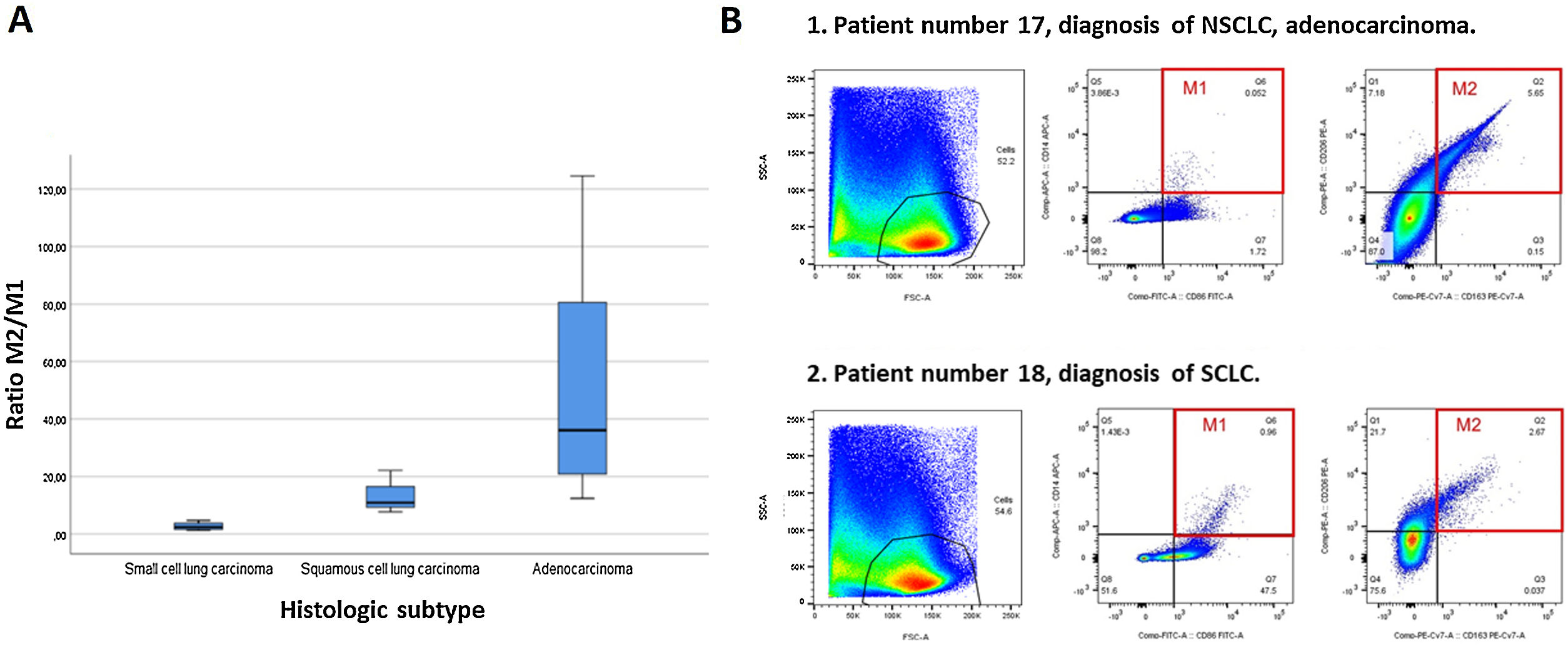
A higher percentage of M2 macrophage population was seen in patients with lung cancer: 1.61 (0.64–5.63) compared to patients with benign lung disease: 0.32 (0.30–0.68), p=0.009. When subanalysis by type of lung cancer was performed, a much higher M2/M1 ratio was obtained in patients with adenocarcinoma: 36.11 (18.43–94.60), 10.89 in squamous cell LC, and 2.41 (1.42–4.25) in SCLC, p=0.005. These results are shown in Fig. 1 and are due to significant differences in the M1 between the different types of lung cancer, much higher in SCLC 0.66 (0.27–4.40), squamous cell LC 0.47 (0.21–0.47) and adenocarcinoma 0.35 (0.11–0.72), p=0.007.
Flow cytometry is the gold standard for phenotyping and quantifying the immune cells.10 Previous studies have shown that increased tumour-associated macrophages (TAMs) density correlates with a poor prognosis in lung cancer.11,12 Nowadays, the notion that TAMs resemble M2 macrophages has been supported in vitro and in vivo.7 TAMs produce cytokines and other proteins to maintain immunosuppression, and a strong correlation has been demonstrated between TAMs and chemotherapy resistance,13 also M1 have shown to enhanced the sensitivity of lung cancer cell A549 to cisplatin and decreased the tube formation activity and cell viability of A549 cells.14 Regarding our results, all samples from lung cancer patients had a higher percentage of M2 macrophages. This is expected as this macrophage subtype favours tumour progression. However, the M2/M1 ratio is not equal for all patients and there is a remarkable difference in the case of patients with SCLC. In a recent study made with 67 patients was found that M2 macrophages significantly increased in patients with SCLC, with simultaneously elevated IL-10, as compared to NSCLC patients.15 Furthermore, the increased frequency of M2 and elevated level of IL-10 in BAL of SCLC patients were positively correlated with advanced tumour stage, but negatively correlated with their survival time.15 We believe that the differences with our results may be due to an only use a specific marker to identify M2 macrophages (CD206), while we have used two (C206 and CD163) and no M1-specific markers are used.
BAL analysis is a promising approach to evaluating the tumour microenvironment. Targeting this tumour-associated macrophages may be at the forefront of lung cancer research and it could be in the future a new treatment strategy.16–18 In fact, an article published in 2018 demonstrated that M1NV-R848 injection can repolarize M2 TAMs to M1 and potentiate antitumor efficacy of the PD-1 checkpoint inhibitor therapy in bladder cancer.19 In our findings, we can see that in patients with SCLC in addition to having high M2 macrophages, they also have high M1, thus making their M2/M1 ratio significantly lower compared to other types of lung cancer. We don’t know if this could be related to its different neuroendocrine cell origin and aggressiveness, or if it could influence its central parahiliar or paramediastinal location (90%), causing atelectasis and retention of secretions distally. Some study limitations should be acknowledged. We have a small sample size ant the control subjects used are not completely healthy. The reliability of the findings and its implications should be confirmed in a larger cohort and over a longer timeframe.
In conclusion, the immunological study has corroborated the preponderance of M2 macrophages in patients with lung cancer. Subanalysis of the data shows that alveolar macrophage expression differs by lung cancer subtype, before receiving any oncological treatment.
Authors’ contributionsVioleta Esteban: investigation; formal analysis; writing – original draft; Javier Javaloyes: investigation; writing – original draft; Sebastián Martínez: conceptualization; investigation; formal analysis; writing – review and editing; José Noberto Sancho: bronchoscopist who obtained study samples; writing – review and editing; Consuelo Ferrer: investigation; methodology; formal analysis; writing – review and editing; Beatriz Gálvez: bronchoscopist who obtained study samples; Eusebi Chiner: conceptualization; supervision; writing – original draft. Maria Francisca Colom: conceptualization; formal analysis; methodology; supervision; writing – original draft.
FundingThis work has received funding from two competitive grants from Fundación Española del Pulmón (SEPAR 2020/1134) and from Fundación de Neumología de la Comunidad Valenciana (SVN-2021/VER).
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to Javier Puentes, laboratory technician, for their help with sample processing and to Ana Pulido and Lía Maestre, bronchoscopy nurses, for their help in ensuring optimal sample collection.