ALAT-2014 COPD Clinical Practice Guidelines used clinical questions in PICO format to compile evidence related to risk factors, COPD screening, disease prognosis, treatment and exacerbations. Evidence reveals the existence of risk factors for COPD other than tobacco, as well as gender differences in disease presentation. It shows the benefit of screening in an at-risk population, and the predictive value use of multidimensional prognostic indexes. In stable COPD, similar benefits in dyspnea, pulmonary function and quality of life are achieved with LAMA or LABA long-acting bronchodilators, whereas LAMA is more effective in preventing exacerbations. Dual bronchodilator therapy has more benefits than monotherapy. LAMA and combination LABA/IC are similarly effective, but there is an increased risk of pneumonia with LABA/IC. Data on the efficacy and safety of triple therapy are scarce. Evidence supports influenza vaccination in all patients and anti-pneumococcal vaccination in patients < 65years of age and/or with severe airflow limitation. Antibiotic prophylaxis may decrease exacerbation frequency in patients at risk. The use of systemic corticosteroids and antibiotics is justified in exacerbations requiring hospitalization and in some patients managed in an outpatient setting.
La guía de práctica clínica de enfermedad pulmonar obstructiva crónica (EPOC) ALAT 2014 fue elaborada contestando preguntas clínicas en formato PICO a través del análisis de evidencias sobre factores de riesgo, búsqueda de casos, evaluación pronóstica, tratamiento y exacerbaciones. La evidencia indica que existen factores de riesgo diferentes al tabaco, diferencias según el género, soporta la búsqueda activa de casos en población de riesgo y el valor predictivo de los índices multidimensionales. En la EPOC estable se encuentran similares beneficios de la monoterapia broncodilatadora (LAMA o LABA) sobre la disnea, función pulmonar o calidad de vida, y mayor efectividad del LAMA para prevenir exacerbaciones. La doble terapia broncodilatadora tiene mayores beneficios comparada con la monoterapia. La eficacia de la terapia con LAMA y la combinación LABA/CI es similar, con mayor riesgo de neumonía con la combinación LABA/CI. Existe limitada información sobre la eficacia y la seguridad de la triple terapia. La evidencia soporta el uso de vacunación contra la influenza en todos los pacientes y contra neumococo en <65años y/o con obstrucción grave. Los antibióticos profilácticos pueden disminuir la frecuencia de exacerbaciones en pacientes de riesgo. Está justificado el uso de corticosteroides sistémicos y antibióticos en exacerbaciones que requieren tratamiento intrahospitalario y en algunas de tratamiento ambulatorio.
The ALAT-2014 guidelines on chronic obstructive pulmonary disease (COPD) are the result of a collaborative project. These recommendations contain regional information and clinical practice guideline (CPG) tools to improve the effectiveness, efficiency and safety of routine treatment decisions related to COPD patients.
This document presents the methodology of the CPG and the development of the PICO format questions formulated in each chapter. The complete version of the CPG is available online.
MethodologyWorking Group and Design of Clinical QuestionsThe working group was formed of members of the 2011 Expert Consensus Group, along with other experts in drafting and/or evaluating CPGs who were invited to join the project. The group was divided into 5 teams to address the following topics:
- •
Methodology
- •
Epidemiology and definition
- •
Diagnosis
- •
Treatment of stable COPD
- •
Exacerbation
The task of these teams was to draw up the clinical questions contained in the guideline.
The questions were formulated in PICO or PECO format: Patient, (Problem or Population), Intervention or Exposure, Comparison and Outcome.1
Two metasearch engines were used for the literature search: Tripdatabase and PubMed. The first was used to establish the hierarchy for the introductory information in each chapter, and to answer the PICO questions; MeSH was used to search PubMed to compare and supplement the search for PICO questions. Table 1 shows the keywords used in the Tripdatabase search and the MeSH terms. The number and type of relevant studies retrieved for each question, shown in Table 2, were evaluated by at least 3 experts, and only those with a Critical Appraisal Skills Program España (CASPE) score of ≥70% were selected. To update the content of each chapter, priority was given to existing guidelines, secondary evidence, extensive primary clinical trials and studies retrieved from Tripdatabase following a keyword-based search strategy.
Search Strategy (Tripdatabase Keywords and MeSH Terms).
| Clinical question | PICO question | Search strategy using MeSH terms |
|---|---|---|
| Are there inhaled substances, other than tobacco smoke, that constitute a risk factor in the development of COPD? | (a) P=COPD or Chronic Obstructive Pulmonary DiseaseI=outdoor air pollution OR occupational exposureC=ØO=Risk factor(a) P=COPD or Chronic Obstructive Pulmonary DiseaseI=secondhand smokeC= ØO=Risk factor(a) P=COPD or Chronic Obstructive Pulmonary DiseaseI=biomassC=ØO=Risk factor | (a) Terms for COPD AND OCCUPATIONAL EXPOSURE AND AIR POLLUTION(“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction AND “Occupational Exposure”[Mesh] AND “Air Pollutants, Occupational”[Mesh] AND (“risk factors”[MeSH Terms] OR risk factors[Text Word] OR factors, risk OR factors, risk OR risk factor))(b) Terms for COPD AND SECOND HAND SMOKING(“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (Passive OR Passive Smoking OR Smoking, Passive OR Secondhand Smoking OR Secondhand Smoking OR Smoking, Secondhand OR Smoking, Secondhand OR Second Hand Smoke OR Hand Smoke, Second OR Hand Smoke, Second OR Second Hand Smoke OR Smoke, Second Hand OR Smokes, Second Hand OR Secondhand Smoke OR Secondhand Smoke OR Smoke, Secondhand OR Smokes, Secondhand OR Involuntary Smoking OR Involuntary Smoking OR Smoking, Involuntary OR Smoking, Involuntary OR Passive Smoking) AND (“risk factors”[MeSH Terms] OR risk factors[Text Word] OR factors, risk OR factors, risk OR risk factor)(c) Terms for COPD AND BIOMASS EXPOSURE(“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction)) AND “Biomass”[Mesh]) AND (“risk factors”[MeSH Terms] OR risk factors[Text Word] OR factors, risk OR factors, risk OR risk factor) |
| Does COPD in women have different epidemiological and clinical characteristics? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=GenderC=ØC=Ø | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction AND “Gender Identity”[Mesh] AND (“gender identity”[MeSH Terms] OR (“gender”[All Fields] AND “identity”[All Fields]) OR “gender identity”[All Fields] OR (“gender”[All Fields] AND “identities”[All Fields]) OR “gender identities”[All Fields]) |
| In groups exposed to risk factors, is active spirometry screening the best strategy for detecting patients with COPD? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=spirometryC=ØO=diagnosis | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (“Mass screening”[MeSH Terms] AND “spirometry”[Mesh terms]) AND Diagnosis”[Mesh terms]) |
| Which multidimensional indexes to predict mortality in COPD have been validated? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=multidimensional index or Severity of IllnessIndexC=ØO=Mortality | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (“Severity of Illness Index”[Mesh] and “mortality”[Mesh]) |
| In COPD patients, do long-acting muscarinic antagonists (LAMAs) offer greater benefit than long-acting β-2 agonists (LABAs)? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=TiotropiumC=Long-acting beta2-agonistO=Ø | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND ((“Adrenergic beta-2 Receptor Agonists”[Mesh]) OR (“indacaterol”[Supplementary Concept] OR 5-AND (2-AND (5,6-diethylindan-2-ylamino) AND-1-hydroxyethyl) AND -8-hydroxy-1H-quinolin-2-one OR QAB-149 OR arcapta neohaler OR Onbrez)) AND ((“Cholinergic Antagonists”[Mesh]) OR (“tiotropium”[Supplementary Concept] OR Spiriva OR tiotropium bromide OR 7- AND ((hydroxybis AND (2-thienyl) AND acetyl) AND oxy) AND-9,9-dimethyl-3-oxa-9-azoniatricyclo AND (3.3.1.0 AND (2,4)) AND nonane bromide OR BA 679 BR OR BA-679 BR)) |
| In COPD patients, does combination LABA+LAMA offer greater benefit than LABA or LAMA monotherapy? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=long-acting beta2-agonist and tiotropiumC=tiotropium OR long-acting beta2-agonistO=Ø | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND ((“tiotropium”[Supplementary Concept] OR Spiriva OR tiotropium bromide OR 7- AND ((hydroxybis AND (2-thienyl) AND acetyl) AND oxy) AND -9,9-dimethyl-3-oxa-9-azoniatricyclo AND (3.3.1.0 AND (2,4)) AND nonane bromide OR BA 679 BR OR BA-679 BR) AND ((“Adrenergic beta-2 Receptor Agonists”[Mesh]) OR (“indacaterol”[Supplementary Concept] OR 5- AND (2- AND (5,6-diethylindan-2-ylamino) AND -1-hydroxyethyl) AND -8-hydroxy-1H-quinolin-2-one OR QAB-149 OR arcapta neohaler OR Onbrez))) |
| In COPD patients, does combined LABA/inhaled corticosteroids (LABA/IC) offer greater benefit than LAMA monotherapy? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=Combination inhaled steroid and long-acting beta2-agonistC=TiotropiumO=Ø | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (((“Adrenergic beta-2 Receptor Agonists”[Mesh]) OR (“indacaterol”[Supplementary Concept] OR 5- AND (2- AND (5,6-diethylindan-2-ylamino) AND -1-hydroxyethyl) AND -8-hydroxy-1H-quinolin-2-one OR QAB-149 OR arcapta neohaler OR Onbrez)) AND (“Glucocorticoids”[Mesh] OR glucocorticoid effect OR effects, glucocorticoid OR glucorticoid effects OR effects, glucorticoid)) AND ((“Cholinergic Antagonists”[Mesh]) OR (“tiotropium”[Supplementary Concept] OR Spiriva OR tiotropium bromide OR 7- AND ((hydroxybis AND (2-thienyl) AND acetyl) AND oxy) AND -9,9-dimethyl-3-oxa-9-azoniatricyclo AND (3.3.1.0 AND (2,4)) AND nonane bromide OR BA 679 BR OR BA-679 BR)) |
| In COPD patients, does combination LABA/IC plus tiotropium (triple therapy) offer greater benefits than tiotropium monotherapy, combination LABA/IC or double bronchodilator therapy (LABA+LAMA)? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=inhaled steroid plus long-acting beta2-agonist plus tiotropiumC=tiotropium OR inhaled steroid and long-acting beta2-agonistO=Ø | (“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND ((“tiotropium”[Supplementary Concept] OR Spiriva OR tiotropium bromide OR 7- AND ((hydroxybis AND (2-thienyl) AND acetyl) AND oxy) AND -9,9-dimethyl-3-oxa-9-azoniatricyclo AND (3.3.1.0 AND (2,4)) AND nonane bromide OR BA 679 BR OR BA-679 BR) AND ((“indacaterol”[Supplementary Concept] OR 5- AND (2- AND (5,6-diethylindan-2-ylamino) AND -1-hydroxyethyl) AND -8-hydroxy-1H-quinolin-2-one OR QAB-149 OR arcapta neohaler OR Onbrez) OR (“Adrenergic beta-2 Receptor Agonists”[Mesh])) AND (“Glucocorticoids”[Mesh] OR glucocorticoid effect OR effects, glucocorticoid OR glucorticoid effects OR effects, glucorticoid)) |
| In COPD patients, is the use of immunization and bacterial lysates justified? | P=COPD or Chronic Obstructive Pulmonary DiseaseI=vaccine or vaccinationC=ØO=exacerbation or hospitalization or mortality | (((“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction)) AND (“Vaccines”[Mesh])) AND ((“Mortality”[Mesh]) OR (“Hospitalization”[Mesh]) OR (“Disease Progression”[Mesh]) OR exacerbation*) |
| In patients with stable COPD, does the use of prophylactic antibiotics prevent exacerbations? | P=COPD exacerbationsI=Prophylactic antibioticsC=ØO=Prevention and Control | ((((“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (acute* OR Exacerbation*))) AND (“prevention and control”[Subheading])) AND (“Anti-Bacterial Agents”[Mesh]) |
| Are antibiotics effective in all COPD exacerbations? | P=COPD exacerbationsI=AntibioticsC=ØO=hospitalization or mortality | (((“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (acute* OR Exacerbation*))) AND (“Anti-Bacterial Agents”[Mesh]) AND (“Mortality”[Mesh] OR “Hospitalization”[Mesh]) |
| Are (oral or parenteral) systemic steroids effective in treating COPD-Es? | P=COPD exacerbationsI=Systemic corticosteroidsC=ØO=Ø | ((((“pulmonary disease, chronic obstructive”[MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction) AND (acute* OR Exacerbation*))) AND (“Glucocorticoids[Mesh] OR glucocorticoid effect OR effects, glucocorticoid OR glucorticoid effects OR effects, glucorticoid)) AND ((systemic[All Fields] OR systemic/10[All Fields] OR systemic/aerosol[All Fields] OR systemic/airway[All Fields] OR systemic/anaphylactic[All Fields] OR systemic/anaphylaxis[All Fields] OR systemic/arterial[All Fields] OR systemic/bladder[All Fields] OR systemic/central[All Fields] OR systemic/cerebral[All Fields] OR systemic/compartmental[All Fields] OR systemic/constitutional[All Fields] OR systemic/core[All Fields] OR systemic/cutaneous[All Fields] OR systemic/disseminated[All Fields] OR systemic/distal[All Fields] OR systemic/environmental[All Fields] OR systemic/extracutaneous[All Fields] OR systemic/familial[All Fields] OR systemic/family[All Fields] OR systemic/general[All Fields] OR systemic/generalized[All Fields] OR systemic/genetic[All Fields] OR systemic/glomerular[All Fields] OR systemic/hepatic[All Fields] OR systemic/historic[All Fields] OR systemic/i[All Fields] OR systemic/immunomodulating[All Fields] OR systemic/inhaled/nebulized[All Fields] OR systemic/intracerebellar[All Fields] OR systemic/intraperitoneal[All Fields] OR systemic/intrasac[All Fields] OR systemic/intrathecal[All Fields] OR systemic/intravenous[All Fields] OR systemic/intravitreal[All Fields] OR systemic/it[All Fields] OR systemic/liver[All Fields] OR systemic/local[All Fields] OR systemic/localized[All Fields] OR systemic/malignant[All Fields] OR systemic/management[All Fields] OR systemic/metabolic[All Fields] OR systemic/metabolic/toxic[All Fields] OR systemic/metastatic[All Fields] OR systemic/microvascular[All Fields] OR systemic/mucosal[All Fields] OR systemic/multiorgan[All Fields] OR systemic/muscular[All Fields] OR systemic/neurologic[All Fields] OR systemic/neurophysiological[All Fields] OR systemic/nonsystemic[All Fields] OR systemic/ocular[All Fields] OR systemic/oral[All Fields] OR systemic/organ[All Fields] OR systemic/organism[All Fields] OR systemic/organizational[All Fields] OR systemic/organs[All Fields] OR systemic/other[All Fields] OR systemic/periocular[All Fields] OR systemic/peripheral[All Fields] OR systemic/portal[All Fields] OR systemic/process[All Fields] OR systemic/productive[All Fields] OR systemic/pulmonary[All Fields] OR systemic/reflective[All Fields] OR systemic/regional[All Fields] OR systemic/remote[All Fields] OR systemic/renal[All Fields] OR systemic/respiratory[All Fields] OR systemic/rheumatic[All Fields] OR systemic/serum[All Fields] OR systemic/strategic[All Fields] OR systemic/structural[All Fields] OR systemic/suprasystemic[All Fields] OR systemic/systems[All Fields] OR systemic/targeted[All Fields] OR systemic/technical[All Fields] OR systemic/tissue[All Fields] OR systemic/topical[All Fields] OR systemic/urticarial[All Fields] OR systemic/vascular[All Fields] OR systemic/vital[All Fields] OR systemic’[All Fields] OR systemica[All Fields] OR systemical[All Fields] OR systemically[All Fields] OR systemically/orally[All Fields] OR systemically’[All Fields] OR systemicautoimmune[All Fields] OR systemicc[All Fields] OR systemicchemotherapy[All Fields] OR systemich[All Fields] OR systemiche[All Fields] OR systemichem[All Fields] OR systemicity[All Fields] OR systemickych[All Fields] OR systemiclupus[All Fields] OR systemicly[All Fields] OR systemicmastocytosis[All Fields] OR systemico[All Fields] OR systemicoportal[All Fields] OR systemicopulmonary[All Fields] OR systemicpulmonary[All Fields] OR systemicresistance[All Fields] OR systemics[All Fields] OR systemicto[All Fields] OR systemicus[All Fields] OR systemicuse[All Fields]) OR Endovenous[All Fields]) |
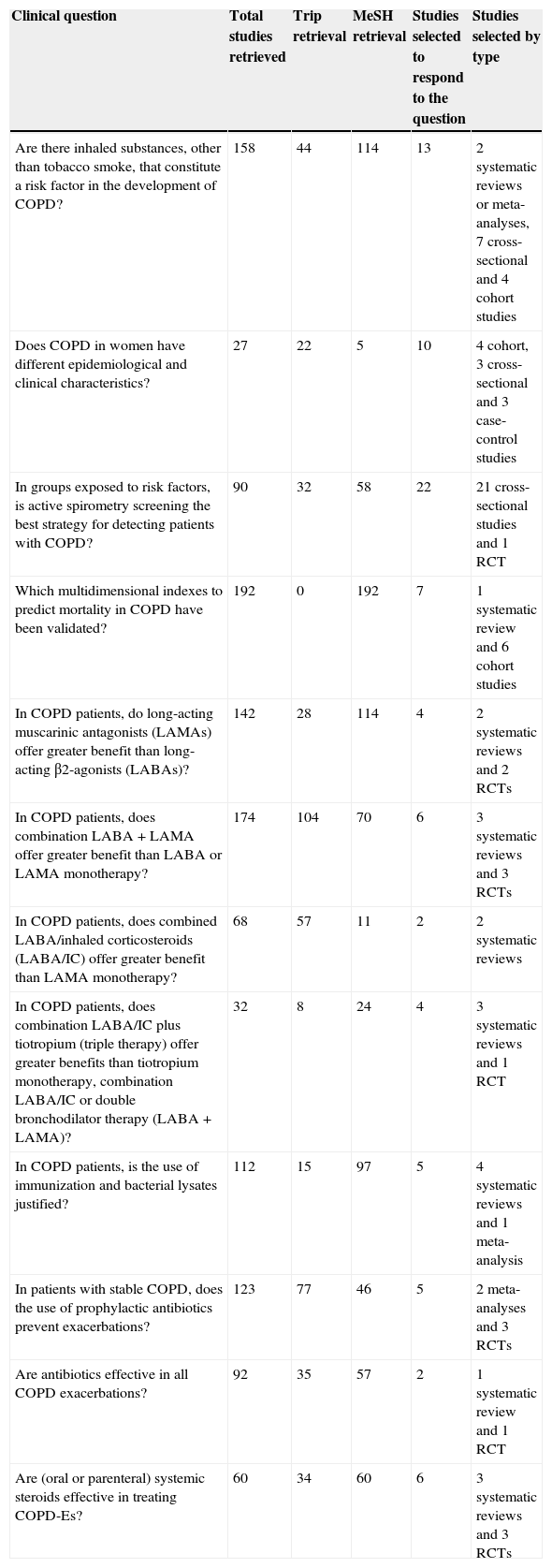
Number and Type of Studies Selected to Respond to Clinical Questions.
| Clinical question | Total studies retrieved | Trip retrieval | MeSH retrieval | Studies selected to respond to the question | Studies selected by type |
|---|---|---|---|---|---|
| Are there inhaled substances, other than tobacco smoke, that constitute a risk factor in the development of COPD? | 158 | 44 | 114 | 13 | 2 systematic reviews or meta-analyses, 7 cross-sectional and 4 cohort studies |
| Does COPD in women have different epidemiological and clinical characteristics? | 27 | 22 | 5 | 10 | 4 cohort, 3 cross-sectional and 3 case-control studies |
| In groups exposed to risk factors, is active spirometry screening the best strategy for detecting patients with COPD? | 90 | 32 | 58 | 22 | 21 cross-sectional studies and 1 RCT |
| Which multidimensional indexes to predict mortality in COPD have been validated? | 192 | 0 | 192 | 7 | 1 systematic review and 6 cohort studies |
| In COPD patients, do long-acting muscarinic antagonists (LAMAs) offer greater benefit than long-acting β2-agonists (LABAs)? | 142 | 28 | 114 | 4 | 2 systematic reviews and 2 RCTs |
| In COPD patients, does combination LABA+LAMA offer greater benefit than LABA or LAMA monotherapy? | 174 | 104 | 70 | 6 | 3 systematic reviews and 3 RCTs |
| In COPD patients, does combined LABA/inhaled corticosteroids (LABA/IC) offer greater benefit than LAMA monotherapy? | 68 | 57 | 11 | 2 | 2 systematic reviews |
| In COPD patients, does combination LABA/IC plus tiotropium (triple therapy) offer greater benefits than tiotropium monotherapy, combination LABA/IC or double bronchodilator therapy (LABA+LAMA)? | 32 | 8 | 24 | 4 | 3 systematic reviews and 1 RCT |
| In COPD patients, is the use of immunization and bacterial lysates justified? | 112 | 15 | 97 | 5 | 4 systematic reviews and 1 meta-analysis |
| In patients with stable COPD, does the use of prophylactic antibiotics prevent exacerbations? | 123 | 77 | 46 | 5 | 2 meta-analyses and 3 RCTs |
| Are antibiotics effective in all COPD exacerbations? | 92 | 35 | 57 | 2 | 1 systematic review and 1 RCT |
| Are (oral or parenteral) systemic steroids effective in treating COPD-Es? | 60 | 34 | 60 | 6 | 3 systematic reviews and 3 RCTs |
RCT, randomized controlled trial.
The studies retrieved for PICO questions were prioritized according to the highest level of evidence (randomized controlled trials [RCTs], meta-analyses and systematic reviews) and the most appropriate answer to the clinical question. Whenever this was not possible, intermediate (observational) or low level (open-label, case series or consensus) studies were selected. The recommended algorithmic selection method was used primarily for therapeutic questions.2 The results of RCTs included in a systematic review are not described separately, unless they address a highly relevant aspect that merits additional observations (for example, secondary outcomes). Studies published in Spanish, Portuguese and English were considered for inclusion. The end date of the search was October 2013.
Critical Analysis and Formulating RecommendationsThe critical appraisal of the studies selected was performed according to the recommendations and templates developed by the CASPE network (www.redcaspe.org). For this purpose, the ACCP grading system was used to classify recommendations as strongor weak according to the balance of benefits, risks, burdens, and possibly cost. The quality of evidence was classified as high, intermediate or low, according to the study design, the consistency of the results, and the ability of the evidence to clearly answer PICO questions. This system was chosen because it is simple, transparent, explicit and consistent with the existing methodological approach to developing evidence-based CPGs.3
A group of external reviewers with experience in COPD was formed. This group is detailed in the ***“authors and contributors” section. The final version of these guidelines has been reviewed and approved by all the authors.
PICO QuestionsThe CPG uses PICO questions to address evidence and controversies relating to risk factors, screening, prognostic evaluation, treatment of stable COPD, prevention and treatment of exacerbations.
Risk FactorsThe importance of risk factors other than smoking in COPD and the influence of patient gender on the disease are still controversial.
1. Question: Are there inhaled substances, other than tobacco smoke, that constitute a risk factor in the development of COPD?
JustificationAlthough smoking is the main risk factor for COPD, a significant number of cases cannot be attributed to this exposure. Other risk factors (exposure to biomass smoke, occupational exposure to dusts and gases, and outdoor air pollution) have been linked to the pathogenesis of COPD.4
Search ResultsA total of 158 studies were retrieved (MeSh: 114, Tripdatabase: 44), of which 13 were selected to respond to the question (2 systematic reviews, 7 cross-sectional studies, and 4 cohort studies).
Summary of the EvidenceNearly 50% of homes worldwide and 90% of rural dwellings use biomass as a source of domestic fuel. The results of a meta-analysis have shown that individuals exposed to biomass smoke are 2.44 times more likely (CI 95%: 1.9–3.33) to develop COPD.5 This exposure was identified as a risk factor in both men and women.5 Another meta-analysis in women living in rural areas shows an association between exposure to biomass smoke and COPD (OR: 2.40; CI 95%: 1.47–3.93).6 Latin American studies have confirmed this association, indicating that respiratory symptoms develop after exposure to 100h/year, and airflow obstruction after 200h/year or >10 years.7
There is scant information on outdoor air pollution and the development of COPD. The results of a cross-sectional study8 and the follow-up of this cohort suggest an association between this exposure and reduced lung function,9 and an attenuation of symptoms when exposure to airborne particulates decreased.10 Other studies have reported an association with hospitalization and increased levels of contamination.11–13
There is evidence to suggest a causal relationship between occupational exposure and development of COPD. A study of the 10-year cumulative incidence of COPD showed that manual work in industry tended to be a risk factor for the disease (OR: 1.78; CI 95%: 0.80–3.97).14 Non-smoking patients with moderate-to-severe COPD reported exposure to organic dusts in the workplace more often than individuals with no obstruction (30.4% vs 23%).15 According to the NHANES-III survey, 31% of cases of COPD among non-smokers can be attributed to occupation exposure.16
One cross-sectional study has indicated that ≥20h/week of passive smoking increased the risk of COPD by 1.18 (CI 95%: 1.0–1.4).17
Conclusions and RecommendationsThere is evidence that inhalation of substances other than tobacco smoke is a risk factor for the development of COPD.
There is a high level of evidence to suggest an association between COPD and occupational and biomass smoke exposure. There is a strong recommendation to explore risk factors associated with inhalation of substances other than tobacco smoke both clinically and epidemiologically.
2. Question: Does COPD in women have different epidemiological and clinical characteristics?
JustificationPossible differences in the clinical expression of COPD in women (impact, clinical characteristics, progression and mortality) are controversial.
Search ResultsA total of 27 studies were retrieved (MeSh: 5, Tripdatabase: 22), of which 10 were selected to respond to the question (4 cohort studies, 3 cross-sectional studies, and 3 case-control studies).
Summary of the EvidenceIn the context of exposure to tobacco smoke, women are more likely to develop COPD at a earlier age, with a greater decline in lung function.18,19 Diagnosis in the subgroup of women is 1.27 times more common (86.0% vs 67.6%; P<.05).20
Studies in the general population and selected groups have shown that women with a similar degrees of airway obstruction present greater dyspnea, anxiety and depression, fewer radiographic signs of emphysema, worse quality of life, and better survival.21–27
Conclusions and RecommendationsThere are differences in the epidemiological and clinical expression of COPD in women. It is important to consider and identify these manifestations and develop appropriate management strategies, particularly in the case of anxiety and depression.
There is high level evidence that confirms gender-based epidemiological and clinical differences in COPD. There is a strong recommendation to consider gender as an important risk factor for COPD and one that impacts on the clinical expression of the disease.
ScreeningThe best strategies for combating underdiagnosis in COPD are still widely debated.
3. Question: In groups exposed to risk factors, is active spirometry screening the best strategy for detecting patients with COPD?
JustificationUnderdiagnosis of COPD is a significant problem.28–32 The main factors associated with underdiagnosis are: younger age, less severe airway obstruction, and few respiratory symptoms.29 One third of patients detected in population studies or primary care screening programs are asymptomatic, and more than 50% have mild symptoms.30,33–35 The absence or mildness of symptoms at this pre-clinical stage of the disease contributes to underdiagnosis.29,31,32
Spirometry abnormalities are often the only evidence of early stage COPD.31,36 There is still no consensus on which population group should be selected for screening and when spirometry should be performed to reduce the incidence of underdiagnosis. Mass spirometry screening programs are not cost-effective. This is because unless the program targets a high-risk population, only a small number of cases will be detected, and the benefits of drug treatment on asymptomatic patients are uncertain.37–39
The cost-effectiveness of organized spirometry screening of the at-risk population is still unclear, as this group also includes a significant number of asymptomatic individuals, or patients with mild disease that would not benefit from therapy.37 Other authors, however, argue that diagnosis at this stage would permit physicians and patients to address risk factors. This would alter the course of the disease, change lifestyles, and give patients the opportunity to understand their condition and to make the best use of available resources.38,40 At-risk individuals can be identified through a simple interview or by means of a structured questionnaire that identifies chronic respiratory symptoms and exposure to risk factors.41
Search ResultsA total of 90 studies were retrieved (MeSh: 58, Tripdatabase: 32), of which 22 were selected to respond to the question (1 RCT and 21 cross-sectional studies).
Summary of the EvidenceThe studies analyzed mainly evaluate screening strategies in terms of prevalence, proportion of new cases detected, and cost.33,35,42–60 None of the studies analyze performance in terms of impact on the disease course, nor on morbidity and mortality.
To identify the at-risk population in the over-40 age group and justify the use of spirometry, some studies base screening on exposure to tobacco smoke, while others do so on respiratory symptoms, or a combination of both.
The application of questionnaires in both the general population (mass screening) or patients seen for whatever cause (opportunistic screening) enabled practitioners to pre-select a higher risk population and improve the diagnostic yield of spirometry49,57 The initial identification of risk though the presence of chronic respiratory symptoms was less successful than the strategy based on the patient's history of exposure to tobacco smoke (10.8% vs 36.3%).59 When risk identification is based on a history of exposure to contaminants (tobacco smoke), the proportion of cases found was higher than the prevalence of COPD in the general population.61 The presence of concomitant symptoms in exposed individuals increases the probability of diagnosis. One study showed that screening for cases of COPD among smokers attending smoking cessation clinics, with or without respiratory symptoms, can give better results than targeting symptomatic smokers in the general population (13.3% vs 10.1%).54 Given that the population in these programs usually has a greater exposure to tobacco, the success of these screening strategies cannot be extrapolated to programs targeting other patient populations, or to opportunistic screening (for example, in primary care).
Conclusions and RecommendationsSpirometry is recommended for detecting cases of COPD in the over-40 age group exposed to risk factors such as smoking (≥10 pack/years), biomass smoke (≥200h/year or ≥10 years) or occupational contaminants, with or without symptoms, among patients visiting a physician for any reason (opportunistic screening) and those that do not (organized screening).
There is a low level of evidence for the diagnostic yield of spirometry screening programs for COPD among the high-risk population. There is a strong recommendation to perform spirometry in patients with respiratory symptoms and exposure to risk factors. There is a weak recommendation to perform spirometry in asymptomatic patients with exposure to risk factors.
Prognostic EvaluationStratifying patients according to severity of disease using simple, easily obtained variables is the first step in determining a prognosis in COPD. Following the introduction of the BODE index,62 other indexes using different variables to assess prognosis have been developed.
4. Question: Which multidimensional indexes to predict mortality in COPD have been validated?
JustificationAlthough lung function decline is associated with morbidity and mortality in COPD, the process can take many different forms, and multidimensional prognostic markers must be identified and validated.63–68 Severity of dyspnea, age, exercise tolerance, body mass index, exacerbations and quality of life, have all been used individually as predictors of all-cause mortality. A more comprehensive approach to COPD calls for multidimensional scales that can contribute to the decision-making process and help establish priorities. Several different COPD prognostic indexes have been put forward, but it is still unclear as which of these is the most accurate predictor of mortality.
Search ResultsA total of 192 studies were retrieved (MeSh: 192), of which 7 were selected to respond to the question (1 systematic review and 6 cohort studies).
Summary of the EvidenceOne systematic review69 describes the accuracy of 8 indexes in predicting mortality: ADO (Age, Dyspnea, Obstruction),64,70 BODE (Body mass index, Obstruction, Dyspnea, Exercise),62 HADO (Health, Activity, Dyspnea, Obstruction),71 DOREMI BOX (Dyspnea, Obstruction, Rate of Exacerbation, Movement exercise Intolerance, Body Mass Index, blood oxygen disturbances),72 PILE (Predicted FEV1, IL-6 and knee Extensor strength),73 e-BODE (BODE plus Exacerbations) and BODEx (Body mass index, Obstruction Dyspnea, and Exacerbations).74 Other indexes evaluation in clinical studies are: DOSE (Dyspnea, Obstruction, Smoking, Exacerbation),75 mBODE (predicted VO2% instead of walk test),76 mDOSE (modified DOSE; exacerbations replaced by quality of life),76 i-BODE (walk test replaced by shuttle test),77 and CART (Classification And Regression Tree).78
All these mortality-prediction indexes have been validated in COPD patients, and have been shown to be as accurate as FEV1 or more so. In the age-adjusted COCOMICS, the BODE index and it variants (e-BODE and BODEx) was shown to be slightly superior.79
Conclusions and RecommendationsEvidence has shown that multidimensional indexes are superior to FEV1 in predicting mortality in COPD. The BODE index and its variants (e-BODE and BODEx) have been shown to be slightly superior.
There is a high level of evidence to confirm the accuracy of COPD multidimensional indexes in predicting mortality. There is a strong recommendation to use validated multidimensional indexes when assessing prognosis in COPD patients.
Treatment of Stable COPDDrug treatment is mainly aimed at reducing symptoms, frequency and severity of exacerbations, and in improving quality of life, lung function and exercise tolerance. An analysis of the evidence relating to the efficacy and safety of certain drugs will guide therapeutic decisions.
5. Question: In COPD patients, do long-acting muscarinic antagonists (LAMAs) offer greater benefit than long-acting β2-agonists (LABAs)?
JustificationBoth LAMAs and LABAs have been shown to be safe and beneficial in some COPD outcomes. Since both therapeutic options are available, it is reasonable to ask if there are any differences between these drugs in terms of safety and efficacy.
Search ResultsA total of 142 studies were retrieved (MeSh: 114, Tripdatabase: 28), of which 4 were selected to respond to the question (2 systematic reviews and 2 RCTs).
Summary of the EvidenceIn terms of efficacy, 2 systematic reviews showed similar improvement in quality of life, dyspnea, activities of daily living and lung function in both drugs.80,81 However, tiotropium was superior to LABAs as a group (indacaterol, salmeterol or formoterol) in reducing the number of COPD-related exacerbations and hospitalizations. There was no difference between the drugs in all-cause hospitalizations and mortality.80,81 With regard to safety, fewer serious adverse events and study withdrawals were reported with tiotropium use.80
One controlled clinical study comparing indacaterol with tiotropium in patients with severe disease showed that both therapies improve lung function, with comparable safety profiles.82 Tiotropium, meanwhile, was more effective in preventing exacerbations.
The increase in cardiovascular mortality with the Respimat® tiotropium inhaler has stirred up much controversy. One systematic review that included 2 studies with the Respimat® tiotropium inhaler reported an increased risk of overall and cardiovascular mortality in the Respimat® arm, and a reduced incidence of both outcomes with combination therapy (salmeterol/fluticasone).83 The TioSpir safety study, however, reports that both doses of Respimat® tiotropium (5 or 2.5μg/day) were comparable to HandiHaler® tiotropium (18μg) in terms of overall mortality.84 This study excluded patients with a history of myocardial infarction in the 6 months prior to the study, hospitalization for class iii–iv heart failure, or those with unstable or potentially fatal arrhythmia requiring re-treatment in the 12 months prior to the study.
No studies comparing indacaterol with new LAMAs such as glycopyrronium, umeclidinium and aclidinium were found.
Conclusions and RecommendationsIn terms of effectiveness, tiotropium and LABAs are equally effective in dyspnea, lung function and quality of life. Tiotropium is more effective than LABAs in reducing the frequency of exacerbations. Both therapeutic options have a similar safely profile.In accordance with the outcome evaluated:
There is a high level of evidence and a strong recommendation for the use of LABAs or LAMAs (with no preference for any particular bronchodilator) to improve dyspnea, quality of life and lung function.
There is a high level of evidence and a strong recommendation to choose LAMAs (tiotropium) over LABAs to reduce the frequency of exacerbations.
6. Question: In COPD patients, does combination LABA+LAMA offer greater benefit than LABA or LAMA monotherapy?
JustificationSeveral different studies have evaluated the efficacy and safety of double bronchodilator therapy (LABA+LAMA) vs LAMA or LABA monotherapy. The addition of a second bronchodilator with a different mechanism of action would be expected to increase the therapeutic benefit. This gives rise to the question of whether adding a second bronchodilator is justified in patients on single bronchodilator therapy that remain symptomatic.
Search ResultsA total of 174 studies were retrieved (MeSh: 70, Tripdatabase: 104), of which 6 were selected to respond to the question (3 systematic reviews and 3 RCTs).
Summary of the EvidenceIn terms of efficacy, one systematic review showed discreet improvement in quality of life with combination tiotropium+LABA vs tiotropium monotherapy in patients with moderate to severe disease.85 Even though the difference was statistically significant (−1.61; IC 95%: −2.93 to −0.29), the confidence interval excludes the clinically important difference (SGRQ −4 units). No differences were observed in other outcomes (hospitalization, mortality, lung function, exacerbations and symptoms). This review did not include studies analyzing the efficacy of double bronchodilator therapy vs single therapy LABAs. The follow-up period of the selected studies was <1 year.
Another systematic review in patients with moderate to very severe disease that includes studies with <6 months follow-up reports superiority of double bronchodilator therapy over monotherapy (tiotropium+LABA vs tiotropium) in terms of quality of life (SGRQ −1.81 units), dyspnea (TDI reduced by >1 point), lung function (pre- and post-bronchodilator FEV1>100ml), and use of rescue medicine, but not in risk of exacerbation.86 Improvements observed in quality of life were not clinically relevant.
One systematic review in patients with moderate to severe disease showed improvement in lung function (FEV1 105ml; CI 95%: 69–142) and dyspnea (TDI 1.50; CI 95%: 1.01–1.99) with tiotropium+formoterol vs tiotropium.87 Seven of the 8 studies analyzed include patients taking inhaled corticosteroids (IC), raising doubts as to whether the findings can be extrapolated overall to patients not taking IC.87
One RCT comparing tiotropium+indacaterol vs tiotropium in patients with moderate to severe disease reports that double bronchodilator therapy offers greater benefit in lung function (FEV1>100ml) over 12 weeks of follow-up.88 Another RCT compares indacaterol+glycopyrronium vs glycopyrronium or tiotropium in patients with severe to very severe disease, with 64 weeks of follow-up.89 Double bronchodilator therapy was shown to offer greater benefit in lung function (FEV1 70–80ml vs glycopyrronium and 60–80ml vs tiotropium), quality of life (SGRQ −1.9 to −2.8 vs glycopyrronium and −1.7 to −3.1 vs tiotropium) and reduced the rate of exacerbations. A subgroup analysis of exacerbations by type showed no difference in the rate of moderate to severe cases. Comparison of double bronchodilator therapy (glycopyrronium+indacaterol), meanwhile, showed greater benefit over placebo, tiotropium, glycopyrronium and indacaterol in lung function, dyspnea and quality of life in patients with moderate to severe disease, although this finding was not clinically relevant.90
In terms of safety, the frequency of adverse effects in double bronchodilator therapy was comparable with that of LABA or LAMA monotherapy.88–90
Conclusions and RecommendationsIn terms of efficacy, in patients with moderate to very severe COPD, double bronchodilator therapy offers greater benefit over monotherapy in dyspnea, lung function and quality of life, but not in the number of exacerbations. Both therapeutic options have a similar safely profile.
There are a highlevel of evidence and a strong recommendation for using double bronchodilator therapy (LABA+LAMA) vs LAMA or LABA in patients with moderate to very severe disease with persistent symptoms or impaired quality of life while receiving bronchodilator monotherapy.
7. Question: In COPD patients, does combined LABA/inhaled corticosteroids (LABA/IC) offer greater benefit than LAMA monotherapy?
JustificationCombination LABA/IC therapy and LAMA monotherapy is frequently used in COPD.91 Tiotropium and combined (LABA/IC) therapy have been shown to benefit dyspnea, quality of life, frequency of exacerbations and hospitalization.92,93 Since both therapeutic options are available, it is reasonable to ask if there are any differences between these drugs in terms of safety and efficacy.2
Search ResultsA total of 68 studies were retrieved (MeSh: 11, Tripdatabase: 57), of which 2 were selected to respond to the question (2 systematic reviews).
Summary of the EvidenceIn terms of efficacy, one systematic review showed that frequency of exacerbations, hospitalization for exacerbations, and quality of life were similar in both therapeutic options.94 Nevertheless, one of the studies included reported a high dropout rate that created an imbalance between groups. Furthermore, patients were not followed up after dropout, and this factor limits to a certain extent the applicability of the findings.95
Another systematic review showed that combination therapy offers a modest benefit in pre-bronchodilator FEV1 (change: 60ml), use of rescue medicine, and quality of life (SGRQ −2.07 units) over tiotropium monotherapy, although these changes were not clinically relevant.86
In terms of safety, there is evidence for an increased risk for pneumonia and serious adverse effects with combination salmeterol/fluticasone vs tiotropium.86,94 No studies comparing drug combinations other than salmeterol/fluticasone vs tiotropium or other LAMAs were found.
Conclusions and RecommendationsIn terms of efficacy, monotherapy with LAMA and combination LABA/IC therapy are comparable. In terms of safety, there is evidence that combination LABA/IC is associated with a greater risk for pneumonia.
There is a high level of evidence for the use of tiotropium or LABA/IC in terms of improving dyspnea, lung function, quality of life, exacerbation frequency and hospitalization for exacerbation. There is a weak recommendation for choosing LAMA over LABA/IC, due to the increased risk of pneumonia with LABA/IC use.
8. Question: In COPD patients, does combination LABA/IC plus tiotropium (triple therapy) offer greater benefits than tiotropium monotherapy, combination LABA/IC or double bronchodilator therapy (LABA+LAMA)?
JustificationIt has been suggested that the addition of tiotropium to combination LABA/IC could reduce exacerbations, hospitalization, and health care costs in patients with moderate to severe COPD. Despite this, the efficacy and safety of triple therapy (LABA/IC+tiotropium) vs monotherapy or double bronchodilator therapy (LABA+LAMA) is still controversial.
Search ResultsA total of 32 studies were retrieved (MeSh: 24, Tripdatabase: 8), of which 4 were selected to respond to the question (3 systematic reviews and 1 RCT).
Summary of the EvidenceOne systematic review, which included only 1 study comparing triple therapy (LABA/IC+tiotropium) vs double bronchodilator therapy (LABA+LAMA), reported no difference in terms of efficacy (quality of life, lung function, all-cause mortality, exacerbations and hospitalizations), frequency of pneumonia, and other adverse events.96 Although this study was methodologically correct, the findings were inconclusive due to the high and uneven rate of dropouts in both study groups (26% in the triple therapy group and 46% in the double bronchodilator therapy group).
A second systematic review comparing triple therapy (LABA/IC+tiotropium), combined therapy (LABA/CI), and monotherapy (tiotropium) showed that triple therapy improved quality of life and lung function.97 The benefit of triple therapy for mortality, frequency of hospitalization and exacerbations, and long-term risk for pneumonia, however, are unclear. This review includes only 1 study comparing triple therapy with combination LABA/IC,98 reporting significant improvement in FEV1 with triple therapy (0.05; CI 95%: 0.00–0.09), with no difference in adverse effects and dropout rate.
Another review comparing triple and monotherapy (tiotropium) found that triple therapy was more beneficial for lung function and quality of life, with no difference between the two options in terms of exacerbations, dyspnea, pneumonia, serious adverse events and withdrawals.86 The findings of an open-label randomized study in triple therapy vs monotherapy (tiotropium) found that triple therapy offered greater benefit in lung function and quality of life in patients with FEV1≤65%, with no difference in frequency of pneumonia and adverse events.99
Conclusions and RecommendationsIn terms of efficacy, triple therapy is slightly more beneficial for lung function and quality of life in patients with moderate to severe disease, and has a safety profile comparable to other therapeutic options (monotherapy with tiotropium or LABA/IC). There is no evidence that triple therapy is superior to double bronchodilator therapy.
There is a high level of evidence, and a weak recommendation for the use of triple therapy in moderate to severe COPD vs LAMA monotherapy (tiotropium) in terms of improved lung function and quality of life.
There is a high level of evidence, and a weak recommendation for the use of triple therapy in moderate to severe COPD vs combined (LABA/IC) therapy in terms of improved lung function.
Prevention and Treatment of ExacerbationsThe aim of therapeutic strategies to treat COPD exacerbations (COPD-E) is to minimize their impact and prevent further episodes.
9. Question: In COPD patients, is the use of immunization and bacterial lysates justified?
JustificationSome treatment guidelines recommend regular immunization against influenza and pneumococcal infection to prevent COPD-E. This, however, could increase treatment costs and cause adverse effects. The use of bacterial lysates in the prevention of COPD is controversial.
Search ResultsA total of 112 studies were retrieved (MeSh: 97, Tripdatabase: 15), of which 5 were selected to respond to the question (4 systematic reviews and 1 meta-analysis).
Summary of the EvidenceIn terms of efficacy, an evaluation of the evidence suggests that, generally speaking, there is only a 76% likelihood that individuals receiving influenza vaccine (trivalent inactivated split-virus) will avoid episodes of acute respiratory infection.100 The effectiveness of the vaccine in COPD patients was 84%, 45% and 85% for mild, moderate or severe disease, respectively. No differences were found in the number of hospitalizations for acute respiratory infection secondary to influenza and the need for mechanical ventilation in patients receiving the vaccine vs placebo.100 Similar findings have been reported in other systematic reviews.101 In terms of safety, incidence of local adverse events (itching or inflammation) was higher among vaccinated patients, although the same was not true of systemic reactions (headache, myalgia, fever, and skin rash).100,101
One systematic review that included studies in pneumococcal vaccines (14- and 23-valent polysaccharide) in COPD patients found no reduction in the risk for pneumococcal pneumonia and cardiovascular or all-cause mortality.102 Neither was there any reduction in the frequency of exacerbations, hospitalizations or visits to the emergency department. The analysis of a clinical trial in patient subgroups shows that pneumonia (pneumococcal or unknown etiology) prevention strategies were effective in 76% of patients aged<65 years, and in 48% of patients with severe obstruction (FEV1<40%).103 In terms of safety, local and systemic effects were comparable to placebo.102
The findings of a meta-analysis and a systematic review showed that bacterial lysates obtained by mechanical lysis were ineffective in preventing COPD-E.104,105 Cutaneous and urologic adverse effects were common in all patients receiving this therapy.
Conclusions and RecommendationsThere is a high level of evidence and a strongrecommendation for the annual use of anti-influenza vaccine in all COPD patients.
There is a highlevel of evidence and a strongrecommendation for the use of anti-pneumococcal vaccine in COPD patients aged<65 years and/or with severe obstruction (FEV1<40%), and a weak recommendation for the generalized use of this vaccine in other COPD patients.
There is a high level of evidence and a strong recommendation against the use of oral bacterial lysates to prevent COPD-E.
10. Question: In patients with stable COPD, does the use of prophylactic antibiotics prevent exacerbations?
JustificationSeveral different studies have evaluated the possibility of reducing the frequency of COPD-E with antibiotic prophylaxis. The earliest studies showed a reduction in the number of days of disability but no change in the frequency of exacerbations. The drawbacks of this therapy were adverse effects and the development of antibiotic resistance.106 Recent studies have explored the usefulness of different regimens of long-term, intermittent antibiotics that are worth analyzing to draw up recommendation on the use of such strategies in preventing COPD-E.
Search ResultsA total of 123 studies were retrieved (MeSh: 46, Tripdatabase: 77), of which 5 were selected to respond to the question (2 meta-analyses and 3 RCTs).
Summary of the EvidenceThe findings of 2 meta-analyses evaluating the use of macrolides show a reduction in the frequency of exacerbations in patients taking these drugs for at least 6 months.107,108 It is important to draw attention to the heterogeneity of the antibiotic regimens and the background inhalation therapy evaluated in studies included in the meta-analysis (<50% of patients received double or triple therapy).
Administration of 250mg erythromycin every 12h for 12 months reduced moderate to severe exacerbations in patients with moderate to severe disease.109 Similarly, administration of 250mg azithromycin per day for 12 months reduced the frequency of exacerbations and improved quality of life.110
In terms of safety, administration of azithromycin is associated with greater macrolide resistance and loss of hearing. This study excluded patients with at-rest tachycardia or those at-risk for corrected QT interval prolongation, and therefore cardiovascular risk is still a controversial issue.110
Moxifloxacin (400mg/day) for 5 days every 8 weeks over a period of 48 weeks did not change the frequency of exacerbations or other outcomes (hospitalization, mortality or quality of life).111 However, it was found to reduce the number of exacerbations in patients with productive cough.
The main adverse events associated with moxifloxacin were gastrointestinal in nature (nausea, vomiting and diarrhea).
Conclusions and RecommendationsThe use of antibiotic prophylaxis may diminish the frequency of COPD-E in patients at high risk for exacerbations, despite treatment with bronchodilators and IC. However, due to the existence of adverse events, the possibility of building antibiotic resistance, and the lack of information on the administration of these regimens for periods longer than 12 months, general recommendations for use cannot be formulated.
There is a high level of evidence of diminished exacerbations with the use of antibiotic prophylaxis. There is a strong recommendation to avoid routine or generalized use of prophylactic antibiotics in COPD due to adverse effects.
11. Question: Are antibiotics effective in all COPD exacerbations?
JustificationThe most common cause of COPD-E is respiratory infection. This accounts for approximately 80% of cases (50%–70% of bacterial origin). The severity of COPD-Es, meanwhile, should be classified according to the level of care required: outpatient, hospitalization in a general ward or in the ICU. The routine indication for antibiotics in all COPD-E cases is controversial.
Search ResultsA total of 92 studies were retrieved (MeSh: 57, Tripdatabase: 35), of which 2 were selected to respond to the question (1 systematic review and 1 RCT).
Summary of the EvidenceOne systematic review estimates the benefit of antibiotics in reducing the risk of treatment failure, duration of hospital stay, and risk for mortality in COPD-E patients admitted to an ICU.112 The use of antibiotics in patients with severe exacerbation admitted to general hospital wards reduced treatment failure but had no effect on duration of hospital stay or mortality. The benefit of antibiotics in COPD-E outpatients remains unclear. No differences in treatment failure, re-admission, and time to first exacerbation were observed in either short (5–7 days) or long (10–14 days) treatment regimens.112
A subgroup analysis in an RCT evaluating outcome predictors in mild to moderate COPD-E found a higher incidence of treatment failure without antibiotics (19.9%) compared to the group taking amoxicillin/clavulanic acid (9.5%).113 The main factors associated with the risk of treatment failure without antibiotics were sputum purulence (OR=6.1; CI 95%: 1.5–25; P=.005) and CRP≥40mg/l (OR=13.4; CI 95%: 4.6–38.8; P<.001). When both these factors were present, the likelihood of failure without antibiotics was 63.7%.113
Conclusions and RecommendationsThe use of antibiotics in COPD-E requiring hospitalization is justified, particularly in patients admitted to the ICU. In mild to moderate COPD-E, antibiotic treatment should be considered in patients with purulent sputum and/or high CPR levels.
These are a high level of evidence and a strongrecommendation for the use of antibiotics in severe to very severe exacerbations.
There are a high level of evidence and a weakrecommendation for antibiotics in mild to moderate exacerbations with purulent sputum and/or high CPR levels.
12. Question: Are (oral or parenteral) systemic steroids effective in treating COPD-Es?
JustificationSystemic steroids have been used to treat both out- and inpatients with COPD-E. There is evidence to support their use, although controversy still surrounds the risk of adverse effects, correct dosage, route of administration and duration of treatment.
Search ResultsA total of 60 studies were retrieved (MeSh: 24, Tripdatabase: 36), of which 6 were selected to respond to the question (3 systematic reviews and 3 RCTs).
Summary of the EvidenceTwo systematic reviews found benefit in the oral or parenteral administration of systemic steroids to reduce the risk of treatment failure, duration of hospital stay, recurrence and re-admission at 30 days, improvement in FEV1, arterial blood gases, and dyspnea in out- or inpatients with COPD-E.114,115
The findings of 3 systematic reviews and 3 RCTs support the use of first-choice oral prednisone (30–60mg/day) for 5–14 days, or equivalent doses of methylprednisolone.115–119 Evidence shows that treatment regimens of 5 and 14 days with 40mg/day prednisone are equally effective. Short (5–7 days) and long (10–14 days) treatment regimens are equally effective. Nevertheless, fewer adverse effects (hyperglycemia and respiratory infections) were found in short regimens.
Both oral and intravenous administration were equally effective; however, parenteral administration was associated with higher costs and local complications.116,118 In terms of safety, the most common adverse event is transient hyperglycemia. In patients with frequent exacerbations requiring repeated steroid regimens, cumulative dose increases the risk of diabetes, osteoporosis, fractures, weight gain, insomnia and ocular complications.119
Conclusions and RecommendationsSystemic steroids should preferably be administered orally at a dose equivalent to 40mg/day prednisone for 5–10 days. They are effective and safe for the treatment of COPD-E. Steroids should be taken orally; parenteral administration should be limited.
These is a high level of evidence and a strong recommendation for the use of oral systemic steroid in COPD-E requiring out- or inpatient treatment.
FundingAstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Novartis and Takeda. The sponsors have not influenced or contributed to any part of these guidelines. None of the authors have received fees for their contribution to this updated version of the guidelines.
Conflict of InterestMaría Montes de Oca has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim and Novartis.
María Victorina López Varela has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, GlaxoSmithKline and Takeda.
Agustín Acuña has received scientific consultancy and/or speaker's fees from AstraZeneca and Novartis.
Eduardo Schiavi has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim and Takeda.
María Alejandra Rey has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim Novartis and Roche.
José Jardim has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, GlaxoSmithKline, MSD, Chiesi, Grifols, CSL, Ache and Teva.
Alejandro Casas has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Novartis, Takeda, Biotoscana and MSD.
Antonio Tokumoto has received scientific consultancy and/or speaker's fees from AstraZeneca and Boehringer Ingelheim.
Carlos A. Torres Duque has received scientific consultancy and/or speaker's fees from Novartis, GlaxoSmithKline, MSD and Biotoscana.
Alejandra Ramírez-Venegas has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline and MSD.
Gabriel García has received scientific consultancy and/or speaker's fees from Novartis, GlaxoSmithKline, Pfizer, MSD and Sanofi.
Roberto Stirbulov has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Bayer, Novartis and GlaxoSmithKline.
Aquiles Camelier has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline and Chiesi.
Miguel Bergna has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline and Sanofi.
Mark Cohen has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline MSD and Oxirent.
Santiago Guzmán has received scientific consultancy and/or speaker's fees from AstraZeneca, Boehringer Ingelheim, Novartis, Bayer, GlaxoSmithKline, Biotoscana, Ferrer Leti, Pfizer and Takeda Nycomed.
Efraín Sánchez declares no conflict of interest.
We would like to thank our external reviewers: Ciro Casanova (Hospital Universitario La Candelaria, Tenerife, Spain), Juan Pablo de Torres (Department of Pulmonary and Respiratory Diseases, Clínica Universitaria de Navarra, Spain) and Rogelio Pérez-Padilla (Instituto Nacional de Enfermedades Respiratorias [INER], Mexico City, Mexico).
Please cite this article as: Montes de Oca M, López Varela MV, Acuña A, Schiavi E, Rey MA, Jardim J, et al. Guía de práctica clínica de la enfermedad pulmonar obstructiva crónica (EPOC) ALAT-2014: Preguntas y respuestas. Arch Bronconeumol. 2015;51:403–416.