Congenital surfactant deficiencies are rare conditions, including mutation in the surfactant protein B (SP-B), surfactant protein C (SP-C) and ABCA3 (ATP-binding cassette member A3) genes. They may present with respiratory failure and pulmonary hypertension (PH) in the newborn. Long-term outcomes are different according to the mutations.
We present an infrequent case, diagnosed in a tertiary hospital, who has survived.
A term female Arabian infant was born via spontaneous vaginal delivery. Mother and father were consanguineous. Immediately after birth, the infant developed respiratory distress and was initially managed with continuous positive airway pressure.
Her physical examination was notable for bilateral coarse breath sounds and generalized thoracic retractions. Chest radiograph demonstrated diffuse bilateral granular opacities. An echocardiogram revealed no evidence of anatomic heart disease with suprasystemic levels of pulmonary artery pressure. Over the next days, her gas exchange worsened, needing intubation and mechanical ventilation. She developed progressive hypoxic respiratory failure that needed high frequency oscillatory ventilation, and nitric oxide administration.
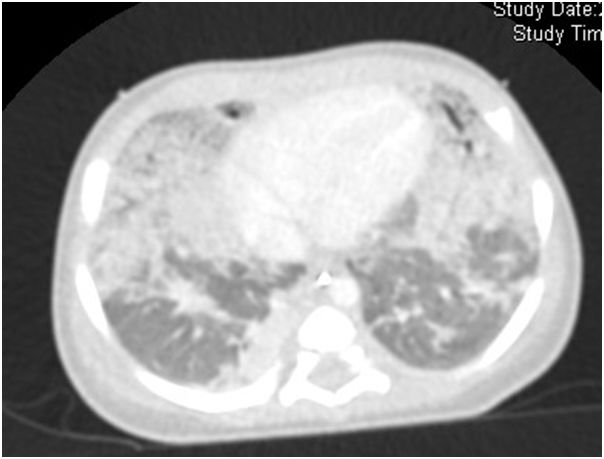
The infant was treated with antibiotics but infectious causes for PH were ruled out with negative blood cultures. Chest computer tomography (Fig. 1) at 15 days of life showed bilateral granular opacities and ground-glass opacification; two doses of surfactant were administered without improvement. Bronchoscopic bronchoalveolar lavage detected PAS positive material. With this information, a lung biopsy trough video thoracoscopy was performed. There were marked alveolar epithelial hyperplasia and mild widening of alveolar walls and the suspicion of a genetic disorder of surfactant dysfunction was considered. She still needed mechanical ventilation and take away a treatment with monthly high intravenous doses of methylprednisolone in association with oral daily hydroxychloroquine and every other day azithromycin. Genetic testing showed a nonsense mutation in ABCA3 gene, c.4681C>T or p.R1561X. This mutation was present on both maternal and paternal alleles.
At 7 months of age the infant was transferred to a pediatric lung transplant unit where she underwent bilateral lung transplantation at 10 months of age. Currently she is 2 years old needing home mechanical ventilation support because of tracheal and right main bronchus malacia.
Interstitial lung diseases (ILD) are a heterogeneous group of pathological processes that affect pulmonary parenchyma and, in most cases, lead to an impairment of gas transfer and reduction of the lung capacity. There are no reliable estimates, but prevalence is likely <1 per 100000.1
The definition requires at least three of the four following criteria in the absence of other know lung disorders: (1) respiratory symptoms (cough, rapid and/or difficult breathing, or exercise intolerance), (2) signs (resting tachypnea, adventitious sounds, retractions, digital clubbing, failure to thrive, or respiratory failure), (3) hypoxemia, and (4) diffuse abnormalities on chest X-ray or CT scan. Thus, establishing 3 of 4 criteria is a sensitivity method for recognizing patients that could benefit from and ILD evaluation.2
The earliest presentation of ILD is shortly after birth, with unexplained respiratory distress in a term neonate.
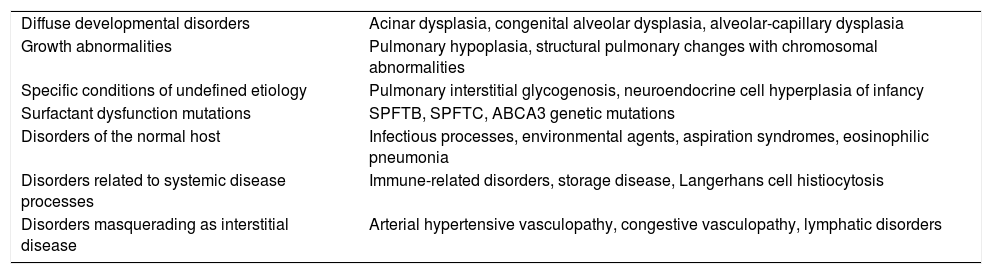
An organized classification scheme for ILD in children less was published by the chILD Research Network3–5 (Table 1).
Classification for ILD in Children.
| Diffuse developmental disorders | Acinar dysplasia, congenital alveolar dysplasia, alveolar-capillary dysplasia |
| Growth abnormalities | Pulmonary hypoplasia, structural pulmonary changes with chromosomal abnormalities |
| Specific conditions of undefined etiology | Pulmonary interstitial glycogenosis, neuroendocrine cell hyperplasia of infancy |
| Surfactant dysfunction mutations | SPFTB, SPFTC, ABCA3 genetic mutations |
| Disorders of the normal host | Infectious processes, environmental agents, aspiration syndromes, eosinophilic pneumonia |
| Disorders related to systemic disease processes | Immune-related disorders, storage disease, Langerhans cell histiocytosis |
| Disorders masquerading as interstitial disease | Arterial hypertensive vasculopathy, congestive vasculopathy, lymphatic disorders |
Given the non-specific presentation of ILD, difficulty is frequently experienced in discerning ILD. Excluding these conditions prior to proceeding to more invasive test is important1,2,6:
- -
Infection screen.
- -
An echocardiography to rule out structural cardiovascular disease and pulmonary hypertension.
- -
Baseline chest X-ray.
- -
Thin-section CT scanning. Ground glass opacification and air trapping are classical features detected.
- -
Flexible bronchoscopy with BAL to exclude infection or airway abnormalities.
- -
Genetic testing. Surfactant protein mutations produce recognizable clinical phenotypes of varying severity.
- -
Lung biopsy is the gold standard.
The gene for ABCA3 is expressed in alveolar type II cells, and the protein is localized to lamellar bodies. ABCA3 mutations have been associated with lethal neonatal respiratory distress and surfactant metabolism dysfunction. Outcomes in patients with ABCA3 mutations are variable, ranging from severe irreversible respiratory failure in early infancy to chronic static or progressive ILD.7,8
There have been no controlled trials of therapeutic interventions in ILD syndrome. Case reports of improvement have been recorded with use of glucocorticoids, hydroxychloroquine, azathioprine, bronchodilators, mycophenolate, and other immune modulators.9,10 Lung transplantation is an option in end-stage lung disease.11
The North American Children study12 found a mortality rate of 30%, with 50% of patients experiencing on-going morbidity. It has become clear that some ILD entities are associated with very high mortality, whereas others have a favorable outcome.