A 4-year-old boy was admitted due to an episode of acute febrile respiratory infection characterized by rhinitis and cough. The patient presented intense dyspnea, tachypnea (60 breaths per minute), bilateral reduction in the vesicular murmur and diffuse crackles. Oxygen saturation (SaO2) while using 4l/min of O2 was 93%, and no response at all was observed to treatment with inhaled beta agonists. The patient was administered clarithromycin, amoxicillin–clavulanic acid and dexamethasone, but his state quickly worsened with increased respiratory effort and the appearance of subcutaneous emphysema. The patient was transferred to the intensive care unit of our pediatric hospital.
The child had been born full term with no postnatal complications. He had been completely healthy up until this episode and had no family history of respiratory diseases.
Upon examination, the patient continued to present dyspnea, and SaO2 was 92% with 7l/min of O2. Laboratory analysis showed mild anemia (10.3g/dl) and a high leukocyte count (18.470/mm3 with 70% of neutrophils). The C-reactive protein, immunoglobulin levels, electrolytes in blood, serum creatinine and liver function tests were normal.
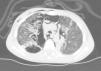
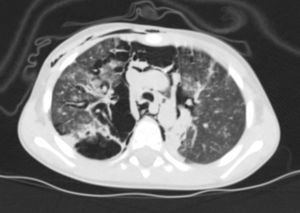
Thoracic radiography revealed thickening of the interstitial pattern, multiple radiotransparent areas due to the interstitial emphysema and parenchymatous opacities in the left middle and lower lung fields. Thoracic computed tomography revealed diffuse pneumomediastinum with subcutaneous emphysema (Fig. 1).
A central venous catheter was inserted for the nutritional support of the patient and for the management of the intravenous treatment. The oxygen supply was maintained at the same time that antibiotic, antifungal, antiviral and anti-inflammatory (high doses of corticosteroids and intravenous immunoglobulin) treatments were initiated empirically while waiting for the lab results. In the hemocultures and cultures of hypopharyngeal aspirate samples, there was no germ growth. Immunodeficiency was ruled out; HIV and CMV tests were negative; sweat test was normal.
During hospitalization, the respiratory state improved and the patient was released 10 days later after a normal physical examination. One week later, a follow-up visit showed the patient to be in a good clinical state, with a normalized acid–base balance and oxygen saturation at 98% breathing room air. On chest radiography, the pneumomediastinum was completely resolved.
At a follow-up visit two years later, the child continued to grow normally. Physical examination showed no signs of significant disease, no nail clubbing was observed and SaO2 was 99% breathing room air. Chest radiography was not done because the parents did not give their consent.
The precedent of an association between gene mutations in surfactant proteins and milder interstitial pneumopathy1–4 led us to evaluate this patient for possible gene mutations associated with surfactant deficiency. The genetic tests for surfactant protein B (SPB) and C (SPC) did not show any variation, but it was observed that the patient was compound heterozygous for two variants of abca3: the substitutions c.863G>A (p.Arg288Lys) and c.875A>T (p.Glu292Val). The Arg288Lys substitution has been previously associated with fatal respiratory distress syndrome (RDS). It was found to affect the first extracellular loop, which is believed to intervene in extracellular interactions5 and therefore exert a serious effect on protein function. The Glu292Val substitution has been erroneously described as being associated with pediatric interstitial pneumopathy.2 Based on the observation of the Glu292Val mutation in many patients with a milder phenotype than what had been seen before, the authors suggested the possibility of a genotype–phenotype correlation. They demonstrated the presence of an alteration in the surfactant metabolism in patients with pediatric interstitial pneumopathy, even when they reached the conclusion that new studies would be necessary about the specific histopathology associated with abca3 mutations for a more exact classification. In addition, other important genetic and environmental variables have been proposed for modifying the course of the disease.
Even when pneumomediastinum is a consequence of asthma crises, it has almost never been reported during acute bronchitis. The first case described of severe pneumomediastinum associated with mutations of the abca3 gene provides new evidence of the phenotypic variability. Currently, we cannot predict the exact correlation between the gene variants, their combined effect or their interaction with other genetic or environmental factors and clinical manifestations. The poor function of the surfactant protein may not only cause milder interstitial pneumopathy in children, but it may also favor alveolar injury, interstitial leak and pneumomediastinum during acute infectious episodes. More data are necessary to better outline the underlying pathogenic mechanism in the clinical spectrum of surfactant deficiency. A better correlation could be obtained between genotype and phenotype using the determination of the incidence of abca3 gene mutations in lung disease.
Please cite this article as: Copertino M, et al. Neumomediastino grave y mutación del gen ABCA3 en un niño: una relación enigmática. Arch Bronconeumol. 2012; 48: 139-40.